当前位置:
X-MOL 学术
›
Isr. J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Acridine Variations for Coordination Chemistry
Israel Journal of Chemistry ( IF 2.3 ) Pub Date : 2020-03-12 , DOI: 10.1002/ijch.202000006 Kevin O. Omolo 1, 2 , John Bacsa 1, 3 , Joseph P. Sadighi 4
Israel Journal of Chemistry ( IF 2.3 ) Pub Date : 2020-03-12 , DOI: 10.1002/ijch.202000006 Kevin O. Omolo 1, 2 , John Bacsa 1, 3 , Joseph P. Sadighi 4
Affiliation
|
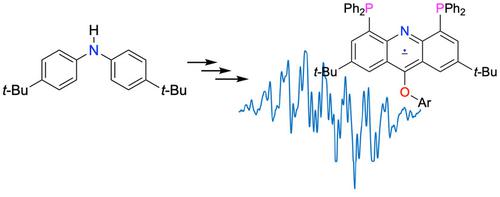
This study describes the synthesis of an acridone through the directed lithiation of a diarylamine, and elaboration via the 4,5‐dibromoacridone. The bromo substituents are amenable to Suzuki‐Miyaura coupling. Conversion to a 9‐chloro‐4,5‐dibromoacridine, followed by bromine‐lithium exchange, allows the preparation of a 4,5‐bis (phosphino)‐9‐chloroacridine. The 9‐chloro moiety undergoes nucleophilic aromatic substitution by methoxide or an aryloxide. One‐electron reduction of a 9‐(aryloxy)‐4,5‐bis(phosphino)acridine affords a stable radical anion.
中文翻译:

配位化学的cr啶变体
这项研究描述了通过直接芳基化二芳基胺和通过4,5-二溴ac啶酮的精制合成one啶酮。溴取代基适合Suzuki-Miyaura偶联。转化为9-氯-4,5-二溴ac啶,然后进行溴-锂交换,可以制备4,5-双(膦基)-9-氯ac啶。9-氯部分经历了甲醇或芳氧基的亲核芳族取代。单电子还原9-(芳氧基)-4,5-双(膦基)ac啶可提供稳定的自由基阴离子。
更新日期:2020-03-12
中文翻译:

配位化学的cr啶变体
这项研究描述了通过直接芳基化二芳基胺和通过4,5-二溴ac啶酮的精制合成one啶酮。溴取代基适合Suzuki-Miyaura偶联。转化为9-氯-4,5-二溴ac啶,然后进行溴-锂交换,可以制备4,5-双(膦基)-9-氯ac啶。9-氯部分经历了甲醇或芳氧基的亲核芳族取代。单电子还原9-(芳氧基)-4,5-双(膦基)ac啶可提供稳定的自由基阴离子。











































 京公网安备 11010802027423号
京公网安备 11010802027423号