当前位置:
X-MOL 学术
›
Isr. J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Pyrrole Functionalization by Copper‐Catalyzed Nitrene Transfer Reactions
Israel Journal of Chemistry ( IF 2.3 ) Pub Date : 2020-03-12 , DOI: 10.1002/ijch.201900181 Anabel M. Rodríguez 1 , Manuel R. Rodríguez 1 , M. Mar Díaz‐Requejo 1 , Pedro J. Pérez 1
Israel Journal of Chemistry ( IF 2.3 ) Pub Date : 2020-03-12 , DOI: 10.1002/ijch.201900181 Anabel M. Rodríguez 1 , Manuel R. Rodríguez 1 , M. Mar Díaz‐Requejo 1 , Pedro J. Pérez 1
Affiliation
|
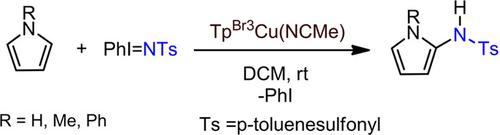
The catalytic functionalization of pyrroles by incorporation of a nitrene group is reported. The Cα‐H bond of 1H‐pyrrole is amidated upon the formal insertion of the NTs (Ts=p‐toluenesulfonyl) group catalyzed by TpBr3Cu(NCMe) (TpBr3=hydrotris(3,4,5‐tribromo‐pyrazolyl)borate). N‐substituted pyrroles also verify the same transformation. The mechanism proposal is similar to that previously described for benzene amidation with the same catalyst and PhI=NTs, which takes place through aziridine formation, ring opening and 1,2‐hydrogen shift. A cascade reaction involving the coupling of 2,5‐dimethylfuran, 1,2,3‐trimethyl‐pyrrole and a nitrene NTs group is also described, leading to a 1,2‐dihydropyridine‐imine compound.
中文翻译:

铜催化的硝基转移反应对吡咯的官能化
据报道,通过引入亚硝基基团,吡咯具有催化功能。的C α -1H-吡咯的-H键时(TS =对甲苯磺酰基)基团由TP催化的NT的正式插入被酰胺化BR3的Cu(NCMe)(TP BR3 =氢三(3,4,5-三溴吡唑基)硼酸)。N-取代的吡咯也证实了相同的转化。机理建议与先前描述的使用相同催化剂和PhI = NTs进行苯酰胺化的方法相似,该方法是通过形成氮丙啶,开环和1,2-氢转移来实现的。还描述了涉及2,5-二甲基呋喃,1,2,3-三甲基吡咯和亚硝基NTs基团偶联的级联反应,从而生成1,2-二氢吡啶-亚胺化合物。
更新日期:2020-03-12
中文翻译:

铜催化的硝基转移反应对吡咯的官能化
据报道,通过引入亚硝基基团,吡咯具有催化功能。的C α -1H-吡咯的-H键时(TS =对甲苯磺酰基)基团由TP催化的NT的正式插入被酰胺化BR3的Cu(NCMe)(TP BR3 =氢三(3,4,5-三溴吡唑基)硼酸)。N-取代的吡咯也证实了相同的转化。机理建议与先前描述的使用相同催化剂和PhI = NTs进行苯酰胺化的方法相似,该方法是通过形成氮丙啶,开环和1,2-氢转移来实现的。还描述了涉及2,5-二甲基呋喃,1,2,3-三甲基吡咯和亚硝基NTs基团偶联的级联反应,从而生成1,2-二氢吡啶-亚胺化合物。










































 京公网安备 11010802027423号
京公网安备 11010802027423号