当前位置:
X-MOL 学术
›
Isr. J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalytically Active Iron(IV)oxo Species Based on a Bis(pyridinyl)phenanthrolinylmethane
Israel Journal of Chemistry ( IF 2.3 ) Pub Date : 2020-03-30 , DOI: 10.1002/ijch.202000009 Henrika M. Hüppe 1 , Kristina Keisers 1 , Fabian Fink 1 , Sonja D. Mürtz 1 , Alexander Hoffmann 1 , Linda Iffland 2 , Ulf-Peter Apfel 2, 3 , Sonja Herres-Pawlis 1
Israel Journal of Chemistry ( IF 2.3 ) Pub Date : 2020-03-30 , DOI: 10.1002/ijch.202000009 Henrika M. Hüppe 1 , Kristina Keisers 1 , Fabian Fink 1 , Sonja D. Mürtz 1 , Alexander Hoffmann 1 , Linda Iffland 2 , Ulf-Peter Apfel 2, 3 , Sonja Herres-Pawlis 1
Affiliation
|
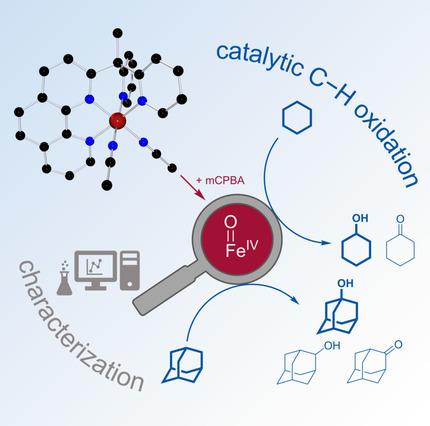
Starting from the mononuclear iron(II) complex [Fe(MeCPy2Phen)(MeCN)2]2+, a non‐heme Fe(IV)oxo complex [FeIV(MeCPy2Phen)O]2+ was synthesized via oxidation with meta‐chloroperoxybenzoic acid (mCPBA). The Fe(IV)oxo complex was characterized using UV/Vis spectroscopy, Mößbauer spectroscopy and CSI mass spectrometry. The ability of this species to oxidize C−H bonds was tested with cyclohexane and adamantane as model substrates. For cyclohexane, an alcohol‐to‐ketone ratio (A/K) of 6.1 and efficiencies up to 55 were obtained. In case of adamantane, the ratio of tertiary over secondary products (3°/2°) is 38. In combination, this indicates the iron(IV)oxo complex being the catalytically active species.
中文翻译:

基于双(吡啶基)菲咯啉基甲烷的催化活性铁(IV)氧物种
从单核铁(II)配合物[Fe(MeCPy 2 Phen)(MeCN)2 ] 2+出发,通过氧化合成非血红素Fe(IV)氧配合物[Fe IV(MeCPy 2 Phen)O] 2+与间-氯过氧苯甲酸(mCPBA)。使用紫外/可见光谱,穆斯堡尔光谱和CSI质谱仪对Fe(IV)羰基配合物进行了表征。以环己烷和金刚烷为模型底物,测试了该物种氧化CH键的能力。对于环己烷,醇与酮之比(A / K)的6.1,效率高达55。在金刚烷的情况下,第三级产物与次级产物的比率(3°/ 2°)为38。综合而言,这表明铁(IV)氧杂络合物是催化活性物质。
更新日期:2020-03-30
中文翻译:

基于双(吡啶基)菲咯啉基甲烷的催化活性铁(IV)氧物种
从单核铁(II)配合物[Fe(MeCPy 2 Phen)(MeCN)2 ] 2+出发,通过氧化合成非血红素Fe(IV)氧配合物[Fe IV(MeCPy 2 Phen)O] 2+与间-氯过氧苯甲酸(mCPBA)。使用紫外/可见光谱,穆斯堡尔光谱和CSI质谱仪对Fe(IV)羰基配合物进行了表征。以环己烷和金刚烷为模型底物,测试了该物种氧化CH键的能力。对于环己烷,醇与酮之比(A / K)的6.1,效率高达55。在金刚烷的情况下,第三级产物与次级产物的比率(3°/ 2°)为38。综合而言,这表明铁(IV)氧杂络合物是催化活性物质。











































 京公网安备 11010802027423号
京公网安备 11010802027423号