当前位置:
X-MOL 学术
›
Helv. Chimica Acta
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Derailed Ohira‐Bestmann Reaction of γ,δ‐Unsaturated Aldehydes for the Stereoselective Synthesis of Cyclopenta[c]pyrazoles
Helvetica Chimica Acta ( IF 1.5 ) Pub Date : 2020-05-18 , DOI: 10.1002/hlca.202000058 Tobias Sandmeier 1 , Niels Sievertsen 1 , Erick M. Carreira 1
Helvetica Chimica Acta ( IF 1.5 ) Pub Date : 2020-05-18 , DOI: 10.1002/hlca.202000058 Tobias Sandmeier 1 , Niels Sievertsen 1 , Erick M. Carreira 1
Affiliation
|
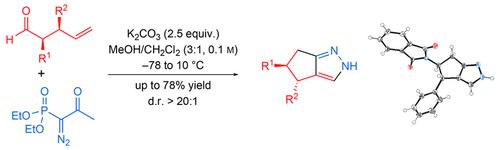
The synthesis of cyclopenta[c]pyrazoles from γ,δ‐unsaturated aldehydes by a domino sequence involving one‐carbon homologation and intramolecular azomethine imine 1,3‐dipolar cycloaddition is disclosed. The fused pyrazoles bearing aromatic and aliphatic substituents are obtained in good yields and excellent diastereomeric purity. Additionally, the synthetic utility of the pyrazole products generated by this method has been highlighted in a series of functionalization reactions. The method presented opens strategic opportunities for the synthesis of pyrazole‐containing biologically active compounds.
中文翻译:

γ,δ-不饱和醛的脱氧Ohira-Bestmann反应用于立体选择合成环戊[c]吡唑
公开了通过涉及一个碳同系物和分子内偶氮甲亚胺亚胺1,3-偶极环加成反应的多米诺序列,由γ,δ-不饱和醛合成环戊[ c ]吡唑。获得具有芳族和脂族取代基的稠合吡唑,具有良好的产率和优异的非对映体纯度。另外,通过该方法产生的吡唑产物的合成效用已经在一系列官能化反应中得到强调。提出的方法为合成含吡唑的生物活性化合物提供了战略机遇。
更新日期:2020-05-18
中文翻译:

γ,δ-不饱和醛的脱氧Ohira-Bestmann反应用于立体选择合成环戊[c]吡唑
公开了通过涉及一个碳同系物和分子内偶氮甲亚胺亚胺1,3-偶极环加成反应的多米诺序列,由γ,δ-不饱和醛合成环戊[ c ]吡唑。获得具有芳族和脂族取代基的稠合吡唑,具有良好的产率和优异的非对映体纯度。另外,通过该方法产生的吡唑产物的合成效用已经在一系列官能化反应中得到强调。提出的方法为合成含吡唑的生物活性化合物提供了战略机遇。











































 京公网安备 11010802027423号
京公网安备 11010802027423号