当前位置:
X-MOL 学术
›
Eur. J. Neurosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Sexual Dimorphism in Stress‐induced Hyperthermia in SNAP25Δ3 mice, a mouse model with disabled Gβγ regulation of the exocytotic fusion apparatus
European Journal of Neuroscience ( IF 3.698 ) Pub Date : 2020-05-25 , DOI: 10.1111/ejn.14836 Analisa D. Thompson Gray 1 , Justice Simonetti 1 , Feyisayo Adegboye 1 , Carrie K. Jones 1, 2 , Zack Zurawski 1, 3 , Heidi E. Hamm 1
European Journal of Neuroscience ( IF 3.698 ) Pub Date : 2020-05-25 , DOI: 10.1111/ejn.14836 Analisa D. Thompson Gray 1 , Justice Simonetti 1 , Feyisayo Adegboye 1 , Carrie K. Jones 1, 2 , Zack Zurawski 1, 3 , Heidi E. Hamm 1
Affiliation
|
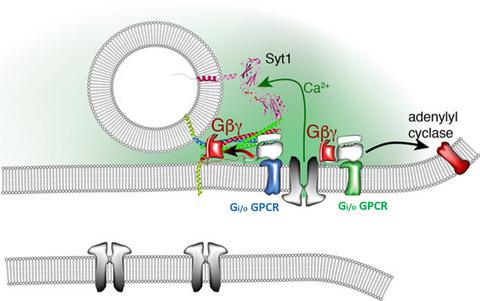
Behavioral assays in the mouse can show marked differences between male and female animals of a given genotype. These differences identified in such preclinical studies may have important clinical implications. We recently made a mouse model with impaired presynaptic inhibition through Gβγ‐SNARE signaling. Here, we examine the role of sexual dimorphism in the severity of the phenotypes of this model, the SNAP25Δ3 mouse. In males, we already reported that SNAP25Δ3 homozygotes demonstrated phenotypes in motor coordination, nociception, spatial memory and stress processing. We now report that while minimal sexually dimorphic effects were observed for the nociceptive, motor or memory phenotypes, large differences were observed in the stress‐induced hyperthermia paradigm, with male SNAP25Δ3 homozygotes exhibiting an increase in body temperature subsequent to handling relative to wild‐type littermates, while no such genotype‐dependent effect was observed in females. This suggests sexually dimorphic mechanisms of Gβγ‐SNARE signaling for stress processing or thermoregulation within the mouse. Second, we examined the effects of heterozygosity with respect to the SNAP25Δ3 mutation. Heterozygote SNAP25Δ3 animals were tested alongside homozygote and wild‐type littermates in all of the aforementioned paradigms and displayed phenotypes similar to wild‐type animals or an intermediate state. From this, we conclude that the SNAP25Δ3 mutation does not behave in an autosomal dominant manner, but rather displays incomplete dominance for many phenotypes.
中文翻译:

SNAP25Δ3小鼠在应激诱导的体温过高中发生性二态性,这是一种胞外融合装置的Gβγ调节功能失效的小鼠模型
小鼠的行为分析可以显示给定基因型的雄性和雌性动物之间的显着差异。在此类临床前研究中发现的这些差异可能具有重要的临床意义。我们最近通过Gβγ-SNARE信号传导建立了一个突触前抑制功能受损的小鼠模型。在这里,我们研究了性二态性在该模型SNAP25Δ3小鼠表型严重性中的作用。在男性中,我们已经报道了SNAP25Δ3纯合子在运动协调,伤害感受,空间记忆和应激处理中表现出表型。我们现在报告,虽然在伤害性,运动或记忆表型方面观察到最小的性二态性影响,但在压力诱导的热疗范例中观察到了很大的差异,与野生型同窝仔相比,雄性SNAP25Δ3纯合子在处理后表现出体温升高,而雌性中没有观察到这种基因型依赖性效应。这表明Gβγ-SNARE信号转导的性双态机制在小鼠体内进行应激处理或温度调节。其次,我们检查了杂合性对SNAP25Δ3突变的影响。在上述所有范式中,杂合子SNAP25Δ3动物与纯合子和野生型同窝动物进行了测试,显示的表型类似于野生型动物或处于中间状态。由此,我们得出结论,SNAP25Δ3突变并不以常染色体显性方式表现,而是对许多表型显示不完全的优势。而在女性中没有观察到这种基因型依赖性效应。这表明Gβγ-SNARE信号转导的性双态机制在小鼠体内进行应激处理或温度调节。其次,我们检查了杂合性对SNAP25Δ3突变的影响。在上述所有范例中,杂合子SNAP25Δ3动物与纯合子和野生型同窝动物进行了测试,表现出与野生型动物或中间状态相似的表型。由此,我们得出结论,SNAP25Δ3突变并不以常染色体显性方式表现,而是对许多表型显示不完全的优势。而在女性中没有观察到这种基因型依赖性效应。这表明Gβγ-SNARE信号转导的性双态机制在小鼠体内进行应激处理或温度调节。其次,我们检查了杂合性对SNAP25Δ3突变的影响。在上述所有范式中,杂合子SNAP25Δ3动物与纯合子和野生型同窝动物进行了测试,显示的表型类似于野生型动物或处于中间状态。由此,我们得出结论,SNAP25Δ3突变并不以常染色体显性方式表现,而是对许多表型显示不完全的优势。我们检查了杂合性对SNAP25Δ3突变的影响。在上述所有范式中,杂合子SNAP25Δ3动物与纯合子和野生型同窝动物进行了测试,显示的表型类似于野生型动物或处于中间状态。由此,我们得出结论,SNAP25Δ3突变并不以常染色体显性方式表现,而是对许多表型显示不完全的优势。我们检查了杂合性对SNAP25Δ3突变的影响。在上述所有范式中,杂合子SNAP25Δ3动物与纯合子和野生型同窝动物进行了测试,显示的表型类似于野生型动物或处于中间状态。由此,我们得出结论,SNAP25Δ3突变并不以常染色体显性方式表现,而是对许多表型显示不完全的优势。
更新日期:2020-06-28
中文翻译:

SNAP25Δ3小鼠在应激诱导的体温过高中发生性二态性,这是一种胞外融合装置的Gβγ调节功能失效的小鼠模型
小鼠的行为分析可以显示给定基因型的雄性和雌性动物之间的显着差异。在此类临床前研究中发现的这些差异可能具有重要的临床意义。我们最近通过Gβγ-SNARE信号传导建立了一个突触前抑制功能受损的小鼠模型。在这里,我们研究了性二态性在该模型SNAP25Δ3小鼠表型严重性中的作用。在男性中,我们已经报道了SNAP25Δ3纯合子在运动协调,伤害感受,空间记忆和应激处理中表现出表型。我们现在报告,虽然在伤害性,运动或记忆表型方面观察到最小的性二态性影响,但在压力诱导的热疗范例中观察到了很大的差异,与野生型同窝仔相比,雄性SNAP25Δ3纯合子在处理后表现出体温升高,而雌性中没有观察到这种基因型依赖性效应。这表明Gβγ-SNARE信号转导的性双态机制在小鼠体内进行应激处理或温度调节。其次,我们检查了杂合性对SNAP25Δ3突变的影响。在上述所有范式中,杂合子SNAP25Δ3动物与纯合子和野生型同窝动物进行了测试,显示的表型类似于野生型动物或处于中间状态。由此,我们得出结论,SNAP25Δ3突变并不以常染色体显性方式表现,而是对许多表型显示不完全的优势。而在女性中没有观察到这种基因型依赖性效应。这表明Gβγ-SNARE信号转导的性双态机制在小鼠体内进行应激处理或温度调节。其次,我们检查了杂合性对SNAP25Δ3突变的影响。在上述所有范例中,杂合子SNAP25Δ3动物与纯合子和野生型同窝动物进行了测试,表现出与野生型动物或中间状态相似的表型。由此,我们得出结论,SNAP25Δ3突变并不以常染色体显性方式表现,而是对许多表型显示不完全的优势。而在女性中没有观察到这种基因型依赖性效应。这表明Gβγ-SNARE信号转导的性双态机制在小鼠体内进行应激处理或温度调节。其次,我们检查了杂合性对SNAP25Δ3突变的影响。在上述所有范式中,杂合子SNAP25Δ3动物与纯合子和野生型同窝动物进行了测试,显示的表型类似于野生型动物或处于中间状态。由此,我们得出结论,SNAP25Δ3突变并不以常染色体显性方式表现,而是对许多表型显示不完全的优势。我们检查了杂合性对SNAP25Δ3突变的影响。在上述所有范式中,杂合子SNAP25Δ3动物与纯合子和野生型同窝动物进行了测试,显示的表型类似于野生型动物或处于中间状态。由此,我们得出结论,SNAP25Δ3突变并不以常染色体显性方式表现,而是对许多表型显示不完全的优势。我们检查了杂合性对SNAP25Δ3突变的影响。在上述所有范式中,杂合子SNAP25Δ3动物与纯合子和野生型同窝动物进行了测试,显示的表型类似于野生型动物或处于中间状态。由此,我们得出结论,SNAP25Δ3突变并不以常染色体显性方式表现,而是对许多表型显示不完全的优势。



























 京公网安备 11010802027423号
京公网安备 11010802027423号