当前位置:
X-MOL 学术
›
Eur. J. Neurosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Roscovitine differentially facilitates cerebellar glutamatergic and GABAergic neurotransmission by enhancing Cav2.1 channel‐mediated multivesicular release
European Journal of Neuroscience ( IF 2.7 ) Pub Date : 2020-05-07 , DOI: 10.1111/ejn.14771 Shin’Ichiro Satake 1, 2 , Shiro Konishi 3
European Journal of Neuroscience ( IF 2.7 ) Pub Date : 2020-05-07 , DOI: 10.1111/ejn.14771 Shin’Ichiro Satake 1, 2 , Shiro Konishi 3
Affiliation
|
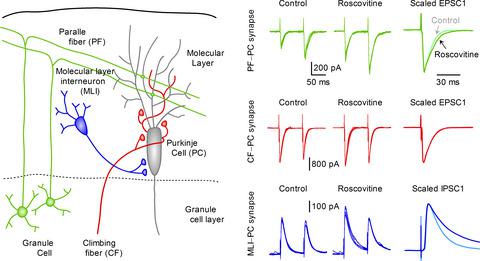
Synaptic vesicle exocytosis is triggered by Ca2+ influx through several subtypes of voltage‐gated calcium channels in the presynaptic terminal. We previously reported that paired‐pulse stimulation at brief intervals increases Cav2.1 (P /Q ‐type) channel‐mediated multivesicular release (MVR) at glutamatergic synapses between granule cells (GCs) and molecular layer interneurons (MLIs) in rat cerebellar slices. However, it has yet to be determined how Cav2 channel subtypes take part in MVR in single axon terminal. This study therefore aimed at examining the effects of roscovitine on different types of cerebellar synapses that make contacts with Purkinje cells (PCs), because this compound has been shown to enhance Cav2.1 channel‐mediated MVR at GC‐MLI synapses. Bath application of roscovitine profoundly increased the amplitude of excitatory postsynaptic currents (EPSCs) at GC‐PC synapses by a presynaptic mechanism as previously observed at GC‐MLI synapses, whereas it caused a marginal effect on climbing fiber‐mediated EPSCs in PCs. At MLI‐PC synapses, roscovitine increased both the amplitude and decay time of inhibitory postsynaptic currents (IPSCs) by enhancing multivesicular GABA release. When extracellular Ca2+ concentration ([Ca2+]e) decreased, roscovitine became less effective in increasing GC‐PC EPSCs. By contrast, roscovitine was able to augment MLI‐PC IPSCs in the low [Ca2+]e. The Cav2.1 channel blocker ω‐agatoxin IVA suppressed the roscovitine‐induced facilitatory actions on both GC‐PC EPSCs and MLI‐PC IPSCs. These results demonstrate that roscovitine enhances MVR at the GC‐PC excitatory synapses in a manner dependent on the driving force of Cav2.1 channel‐mediated Ca2+ influx into the nerve terminal, while it also facilitates MLI‐PC inhibitory transmission via Ca2+‐insensitive mechanisms.
中文翻译:

Roscovitine通过增强Cav2.1通道介导的多囊泡释放来差异性地促进小脑谷氨酸能和GABA能神经传递
Ca 2+通过突触前末端的几种电压门控钙通道亚型流入,触发突触小泡胞吐。我们先前曾报道,短暂间隔的成对脉冲刺激在大鼠小脑切片中的颗粒细胞(GCs)与分子层中神经元(MLIs)之间的谷氨酸能突触处增加Ca v 2.1(P / Q型)通道介导的多囊泡释放(MVR)。 。然而,尚未确定Ca v2个通道子类型在单个轴突终端中参与MVR。因此,本研究旨在检查罗斯科维汀对与浦肯野细胞(PC)接触的不同类型小脑突触的影响,因为该化合物已显示出在GC-MLI突触中增强Ca v 2.1通道介导的MVR。如先前在GC‐MLI突触中观察到的那样,浴液中使用roscovitine的前突触机制极大地增加了GC‐PC突触处的兴奋性突触后突触电流(EPSC)的幅度,而对PC上的纤维介导的EPSC攀爬造成了边际影响。在MLI-PC突触中,roscovitine通过增强多囊性GABA的释放而增加了抑制性突触后电流(IPSC)的幅度和衰减时间。当细胞外Ca 2+浓度([Ca2+ ] e)降低,roscovitine在增加GC-PC EPSC方面变得无效。相比之下,罗斯科维汀能够在低[Ca 2+ ] e下增加MLI-PC IPSC 。Ca v 2.1通道阻滞剂ω-毒素IVA抑制了roscovitine对GC-PC EPSC和MLI-PC IPSC的促进作用。这些结果表明,roscovitine依赖于通过Ca v 2.1通道介导的Ca 2+流入神经末梢的驱动力来增强GC-PC兴奋性突触的MVR ,同时也促进了通过Ca 2的MLI-PC抑制性传递。+-不敏感的机制。
更新日期:2020-06-27
中文翻译:

Roscovitine通过增强Cav2.1通道介导的多囊泡释放来差异性地促进小脑谷氨酸能和GABA能神经传递
Ca 2+通过突触前末端的几种电压门控钙通道亚型流入,触发突触小泡胞吐。我们先前曾报道,短暂间隔的成对脉冲刺激在大鼠小脑切片中的颗粒细胞(GCs)与分子层中神经元(MLIs)之间的谷氨酸能突触处增加Ca v 2.1(P / Q型)通道介导的多囊泡释放(MVR)。 。然而,尚未确定Ca v2个通道子类型在单个轴突终端中参与MVR。因此,本研究旨在检查罗斯科维汀对与浦肯野细胞(PC)接触的不同类型小脑突触的影响,因为该化合物已显示出在GC-MLI突触中增强Ca v 2.1通道介导的MVR。如先前在GC‐MLI突触中观察到的那样,浴液中使用roscovitine的前突触机制极大地增加了GC‐PC突触处的兴奋性突触后突触电流(EPSC)的幅度,而对PC上的纤维介导的EPSC攀爬造成了边际影响。在MLI-PC突触中,roscovitine通过增强多囊性GABA的释放而增加了抑制性突触后电流(IPSC)的幅度和衰减时间。当细胞外Ca 2+浓度([Ca2+ ] e)降低,roscovitine在增加GC-PC EPSC方面变得无效。相比之下,罗斯科维汀能够在低[Ca 2+ ] e下增加MLI-PC IPSC 。Ca v 2.1通道阻滞剂ω-毒素IVA抑制了roscovitine对GC-PC EPSC和MLI-PC IPSC的促进作用。这些结果表明,roscovitine依赖于通过Ca v 2.1通道介导的Ca 2+流入神经末梢的驱动力来增强GC-PC兴奋性突触的MVR ,同时也促进了通过Ca 2的MLI-PC抑制性传递。+-不敏感的机制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号