Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enhanced Photocatalytic H2 -Production Activity of CdS Quantum Dots Using Sn2+ as Cocatalyst under Visible Light Irradiation.
Small ( IF 13.0 ) Pub Date : 2020-06-02 , DOI: 10.1002/smll.202001024 Xianglin Xiang 1 , Bicheng Zhu 1 , Bei Cheng 1 , Jiaguo Yu 1, 2 , Hongjin Lv 3
Small ( IF 13.0 ) Pub Date : 2020-06-02 , DOI: 10.1002/smll.202001024 Xianglin Xiang 1 , Bicheng Zhu 1 , Bei Cheng 1 , Jiaguo Yu 1, 2 , Hongjin Lv 3
Affiliation
|
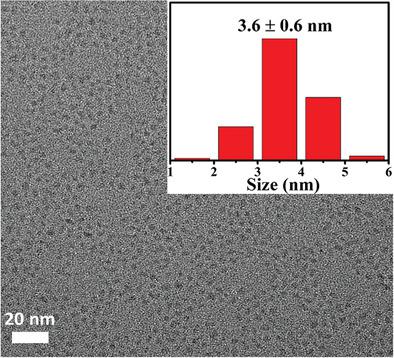
Herein, oil‐soluble CdS quantum dots (QDs) are first prepared through a solvent‐thermal process. Then, oil‐soluble CdS QDs are changed into water‐soluble QDs via ligand exchange using mercaptopropionic acid as capping agent at pH 13. The photocatalytic performance is investigated under the visible light irradiation using glycerol as sacrificial agent and Sn2+ as cocatalyst. No H2‐production activity is observed for oil‐soluble CdS QDs. Water‐soluble CdS QDs exhibit significantly enhanced hydrogen evolution rate. When the concentration of cocatalyst Sn2+ increases to 0.2 × 10−3 m , the rate of hydrogen evolution reaches 1.61 mmol g−1 h−1, which is 24 times higher than that of the pristine water‐soluble CdS QDs. The enhanced H2‐production efficiency is attributed to the adsorption of Sn2+ ions on the surface of CdS QDs that are further reduced to Sn atoms by photogenerated electrons. The in situ generated Sn atoms serve as photocatalytic cocatalyst for efficient hydrogen generation.
中文翻译:

Sn2 +作为助催化剂在可见光照射下增强的CdS量子点的H2催化生产H2活性。
本文首先通过溶剂热法制备油溶性CdS量子点(QD)。然后,通过巯基丙酸作为封端剂在pH 13下通过配体交换将油溶性CdS QDs转变为水溶性QDs。在可见光照射下,以甘油为牺牲剂,Sn 2+为助催化剂,研究了光催化性能。对于油溶性CdS QD,未观察到H 2产生活性。水溶性CdS量子点具有显着提高的析氢速率。当助催化剂Sn 2+的浓度增加到0.2×10 -3 m时,氢的释放速率达到1.61 mmol g -1 h -1,这是原始水溶性CdS QD的24倍。H 2产生效率的提高归因于CdS量子点表面上Sn 2+离子的吸附,而后者通过光生电子进一步还原为Sn原子。原位产生的Sn原子用作光催化助催化剂,可有效产生氢。
更新日期:2020-07-02
中文翻译:

Sn2 +作为助催化剂在可见光照射下增强的CdS量子点的H2催化生产H2活性。
本文首先通过溶剂热法制备油溶性CdS量子点(QD)。然后,通过巯基丙酸作为封端剂在pH 13下通过配体交换将油溶性CdS QDs转变为水溶性QDs。在可见光照射下,以甘油为牺牲剂,Sn 2+为助催化剂,研究了光催化性能。对于油溶性CdS QD,未观察到H 2产生活性。水溶性CdS量子点具有显着提高的析氢速率。当助催化剂Sn 2+的浓度增加到0.2×10 -3 m时,氢的释放速率达到1.61 mmol g -1 h -1,这是原始水溶性CdS QD的24倍。H 2产生效率的提高归因于CdS量子点表面上Sn 2+离子的吸附,而后者通过光生电子进一步还原为Sn原子。原位产生的Sn原子用作光催化助催化剂,可有效产生氢。











































 京公网安备 11010802027423号
京公网安备 11010802027423号