当前位置:
X-MOL 学术
›
Microbiologyopen
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Identification and functional characterization of NAD(P)+ -dependent meso-diaminopimelate dehydrogenase from Numidum massiliense.
MicrobiologyOpen ( IF 3.9 ) Pub Date : 2020-06-02 , DOI: 10.1002/mbo3.1059 Hironaga Akita 1 , Yusuke Nakamichi 1 , Tomotake Morita 2 , Akinori Matsushika 1, 3
MicrobiologyOpen ( IF 3.9 ) Pub Date : 2020-06-02 , DOI: 10.1002/mbo3.1059 Hironaga Akita 1 , Yusuke Nakamichi 1 , Tomotake Morita 2 , Akinori Matsushika 1, 3
Affiliation
|
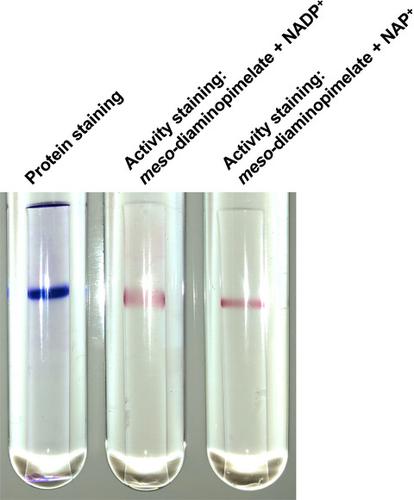
meso‐Diaminopimelate dehydrogenase (meso‐DAPDH) catalyzes the reversible NADP+‐dependent oxidative deamination of meso‐2,6‐diaminopimelate (meso‐DAP) to produce l‐2‐amino‐6‐oxopimelate. Moreover, d‐amino acid dehydrogenase (d‐AADHs) derived from protein‐engineered meso‐DAPDH is useful for one‐step synthesis of d‐amino acids with high optical purity. Here, we report the identification and functional characterization of a novel NAD(P)+‐dependent meso‐DAPDH from Numidum massiliense (NmDAPDH). After the gene encoding the putative NmDAPDH was expressed in recombinant Escherichia coli cells, the enzyme was purified 4.0‐fold to homogeneity from the crude extract through five purification steps. Although the previously known meso‐DAPDHs use only NADP+ as a coenzyme, NmDAPDH was able to use both NADP+ and NAD+ as coenzymes. When NADP+ was used as a coenzyme, NmDAPDH exhibited an approximately 2 times higher kcat/Km value toward meso‐DAP than that of meso‐DAPDH from Symbiobacterium thermophilum (StDAPDH). NmDAPDH also catalyzed the reductive amination of corresponding 2‐oxo acids to produce acidic d‐amino acids such as d‐aspartate and d‐glutamate. The optimum pH and temperature for the oxidative deamination of meso‐DAP were about 10.5 and 75°C, respectively. Like StDAPDH, NmDAPDH exhibited high stability: it retained more than 75% of its activity after 30 min at 60°C (pH 7.2) or at pHs ranging from 5.5 to 13.0 (50°C). Alignment of the amino acid sequences of NmDAPDH and the known meso‐DAPDHs suggested NmDAPDH has a hexameric structure. Given its specificity for both NADP+ and NAD+, high stability, and a broad range of reductive amination activity toward 2‐oxo acids, NmDAPDH appears to offer advantages for engineering a more effective d‐AADH.
中文翻译:

麻疯树NAD(P)+依赖的介观二氨基己二酸酯脱氢酶的鉴定和功能表征。
内消旋-Diaminopimelate脱氢酶(内消旋-DAPDH)催化可逆NADP + -依赖性氧化脱氨的内消旋-二氨基庚二酸-2,6-(内消旋-DAP),以产生升-2-氨基-6- oxopimelate。此外,衍生自蛋白质工程化介观DAPDH的d-氨基酸脱氢酶(d - AADHs)可用于一步合成具有高光学纯度的d-氨基酸。在这里,我们报告从Namimidum massiliense的新型NAD(P)+依赖于介观DADAH的鉴定和功能表征(NmDAPDH)。在重组大肠杆菌细胞中表达编码假定的NmDAPDH的基因后,通过五个纯化步骤从粗提物中将酶纯化4.0倍至同质。尽管以前已知的内消旋DAPDH仅使用NADP +作为辅酶,但NmDAPDH能够同时使用NADP +和NAD +作为辅酶。当NADP +用作辅酶时,NmDAPDH表现出的中观-DAP的k cat / K m值比嗜热共生菌的中观-DAPDH高约2倍。(StDAPDH)。NmDAPDH还催化相应的2-氧代酸的还原胺化反应,生成酸性d-氨基酸,例如d-天冬氨酸和d-谷氨酸。meso- DAP氧化脱氨的最佳pH和温度分别约为10.5和75°C。与StDAPDH一样,NmDAPDH表现出高稳定性:在60°C(pH 7.2)或5.5至13.0(50°C)的pH下30分钟后,它保留了其活性的75%以上。NmDAPDH的氨基酸序列与已知的内消旋DAPDH的比对表明NmDAPDH具有六聚体结构。鉴于其对NADP +和NAD +的特异性NmDAPDH具有较高的稳定性,较高的稳定性以及对2-氧代酸的广泛还原胺化活性,因此为设计更有效的d- AADH提供了优势。
更新日期:2020-06-02
中文翻译:

麻疯树NAD(P)+依赖的介观二氨基己二酸酯脱氢酶的鉴定和功能表征。
内消旋-Diaminopimelate脱氢酶(内消旋-DAPDH)催化可逆NADP + -依赖性氧化脱氨的内消旋-二氨基庚二酸-2,6-(内消旋-DAP),以产生升-2-氨基-6- oxopimelate。此外,衍生自蛋白质工程化介观DAPDH的d-氨基酸脱氢酶(d - AADHs)可用于一步合成具有高光学纯度的d-氨基酸。在这里,我们报告从Namimidum massiliense的新型NAD(P)+依赖于介观DADAH的鉴定和功能表征(NmDAPDH)。在重组大肠杆菌细胞中表达编码假定的NmDAPDH的基因后,通过五个纯化步骤从粗提物中将酶纯化4.0倍至同质。尽管以前已知的内消旋DAPDH仅使用NADP +作为辅酶,但NmDAPDH能够同时使用NADP +和NAD +作为辅酶。当NADP +用作辅酶时,NmDAPDH表现出的中观-DAP的k cat / K m值比嗜热共生菌的中观-DAPDH高约2倍。(StDAPDH)。NmDAPDH还催化相应的2-氧代酸的还原胺化反应,生成酸性d-氨基酸,例如d-天冬氨酸和d-谷氨酸。meso- DAP氧化脱氨的最佳pH和温度分别约为10.5和75°C。与StDAPDH一样,NmDAPDH表现出高稳定性:在60°C(pH 7.2)或5.5至13.0(50°C)的pH下30分钟后,它保留了其活性的75%以上。NmDAPDH的氨基酸序列与已知的内消旋DAPDH的比对表明NmDAPDH具有六聚体结构。鉴于其对NADP +和NAD +的特异性NmDAPDH具有较高的稳定性,较高的稳定性以及对2-氧代酸的广泛还原胺化活性,因此为设计更有效的d- AADH提供了优势。









































 京公网安备 11010802027423号
京公网安备 11010802027423号