当前位置:
X-MOL 学术
›
J. Cell. Physiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ten-year update of the international registry on cytokine-induced killer cells in cancer immunotherapy.
Journal of Cellular Physiology ( IF 4.5 ) Pub Date : 2020-06-02 , DOI: 10.1002/jcp.29827 Ying Zhang 1 , Ingo G H Schmidt-Wolf 1
Journal of Cellular Physiology ( IF 4.5 ) Pub Date : 2020-06-02 , DOI: 10.1002/jcp.29827 Ying Zhang 1 , Ingo G H Schmidt-Wolf 1
Affiliation
|
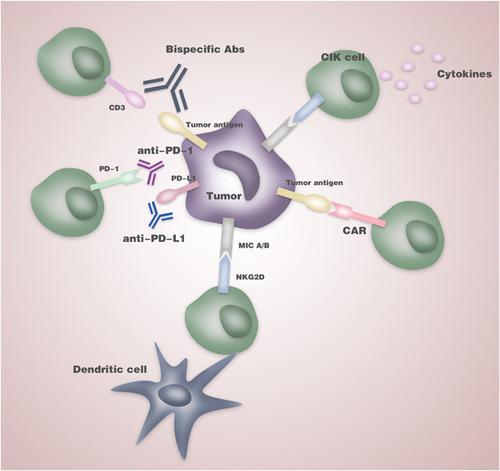
Cytokine‐induced killer (CIK) cells represent an exceptional T‐cell population uniting a T cell and natural killer cell‐like phenotype in their terminally differentiated CD3+CD56+ subset, which features non‐MHC‐restricted tumor‐killing activity. CIK cells have provided encouraging results in initial clinical studies and revealed synergistic antitumor effects when combined with standard therapeutic procedures. We established the international registry on CIK cells (IRCC) to collect and evaluate clinical trials for the treatment of cancer patients in 2010. Moreover, our registry set new standards on the reporting of results from clinical trials using CIK cells. In the present update, a total of 106 clinical trials including 10,225 patients were enrolled in IRCC, of which 4,889 patients in over 30 distinct tumor entities were treated with CIK cells alone or in combination with conventional or novel therapies. Significantly improved median progression‐free survival and overall survival were shown in 27 trials, and 9 trials reported a significantly increased 5‐year survival rate. Mild adverse effects and graft‐versus‐host diseases were also observed in the studies. Recently, more efforts have been put into the improvement of antitumoral efficacy by CIK cells including the administration of immune checkpoint inhibitors and modification with chimeric antigen receptorc. The minimal toxicity and multiple improvements on their tumor‐killing activity both make CIK cells a favorable therapeutic tool in the clinical practice of cancer immunotherapy.
中文翻译:

癌症免疫治疗中细胞因子诱导的杀伤细胞国际注册十年更新。
细胞因子诱导的杀伤细胞(CIK)代表了一个非凡的T细胞群,在其终末分化的CD3 + CD56 +中将T细胞和自然杀伤细胞样表型结合在一起子集,具有非MHC限制的杀肿瘤活性。CIK细胞在最初的临床研究中提供了令人鼓舞的结果,并在与标准治疗程序结合后显示出协同抗肿瘤作用。我们在2010年建立了CIK细胞国际注册中心(IRCC),以收集和评估治疗癌症患者的临床试验。此外,我们的注册中心在报告使用CIK细胞进行临床试验的结果方面设定了新的标准。在本更新中,总共有106个临床试验纳入IRCC,包括10,225例患者,其中30多个不同肿瘤实体中的4,889例患者单独或联合常规或新型疗法治疗了CIK细胞。27个试验显示,无进展生存率和总体生存率显着提高,9个试验报告了5年生存率显着提高。在研究中还观察到轻度的不良反应和移植物抗宿主病。近来,已经在CIK细胞的抗肿瘤功效的改善上进行了更多的努力,包括施用免疫检查点抑制剂和用嵌合抗原受体c修饰。CIK细胞的最小毒性和多种杀伤活性都使CIK细胞成为癌症免疫疗法临床实践中的有利治疗工具。CIK细胞已在改善抗肿瘤功效方面做出了更多努力,包括给予免疫检查点抑制剂和用嵌合抗原受体进行修饰。CIK细胞具有最小的毒性和对肿瘤杀灭活性的多重改进,都使CIK细胞成为癌症免疫疗法临床实践中的有利治疗工具。CIK细胞已在改善抗肿瘤功效方面做出了更多努力,包括给予免疫检查点抑制剂和用嵌合抗原受体进行修饰。CIK细胞具有最小的毒性和对肿瘤杀灭活性的多重改进,都使CIK细胞成为癌症免疫疗法临床实践中的有利治疗工具。
更新日期:2020-06-02
中文翻译:

癌症免疫治疗中细胞因子诱导的杀伤细胞国际注册十年更新。
细胞因子诱导的杀伤细胞(CIK)代表了一个非凡的T细胞群,在其终末分化的CD3 + CD56 +中将T细胞和自然杀伤细胞样表型结合在一起子集,具有非MHC限制的杀肿瘤活性。CIK细胞在最初的临床研究中提供了令人鼓舞的结果,并在与标准治疗程序结合后显示出协同抗肿瘤作用。我们在2010年建立了CIK细胞国际注册中心(IRCC),以收集和评估治疗癌症患者的临床试验。此外,我们的注册中心在报告使用CIK细胞进行临床试验的结果方面设定了新的标准。在本更新中,总共有106个临床试验纳入IRCC,包括10,225例患者,其中30多个不同肿瘤实体中的4,889例患者单独或联合常规或新型疗法治疗了CIK细胞。27个试验显示,无进展生存率和总体生存率显着提高,9个试验报告了5年生存率显着提高。在研究中还观察到轻度的不良反应和移植物抗宿主病。近来,已经在CIK细胞的抗肿瘤功效的改善上进行了更多的努力,包括施用免疫检查点抑制剂和用嵌合抗原受体c修饰。CIK细胞的最小毒性和多种杀伤活性都使CIK细胞成为癌症免疫疗法临床实践中的有利治疗工具。CIK细胞已在改善抗肿瘤功效方面做出了更多努力,包括给予免疫检查点抑制剂和用嵌合抗原受体进行修饰。CIK细胞具有最小的毒性和对肿瘤杀灭活性的多重改进,都使CIK细胞成为癌症免疫疗法临床实践中的有利治疗工具。CIK细胞已在改善抗肿瘤功效方面做出了更多努力,包括给予免疫检查点抑制剂和用嵌合抗原受体进行修饰。CIK细胞具有最小的毒性和对肿瘤杀灭活性的多重改进,都使CIK细胞成为癌症免疫疗法临床实践中的有利治疗工具。










































 京公网安备 11010802027423号
京公网安备 11010802027423号