当前位置:
X-MOL 学术
›
Biotechnol. Bioeng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Role of glycine 256 residue in improving the catalytic efficiency of mutant endoglucanase of family 5 glycoside hydrolase from Bacillus amyloliquefaciens SS35.
Biotechnology and Bioengineering ( IF 3.5 ) Pub Date : 2020-06-02 , DOI: 10.1002/bit.27448 Shweta Singh 1, 2 , Krishan Kumar 1 , Priyanka Nath 1, 2 , Arun Goyal 1, 2
Biotechnology and Bioengineering ( IF 3.5 ) Pub Date : 2020-06-02 , DOI: 10.1002/bit.27448 Shweta Singh 1, 2 , Krishan Kumar 1 , Priyanka Nath 1, 2 , Arun Goyal 1, 2
Affiliation
|
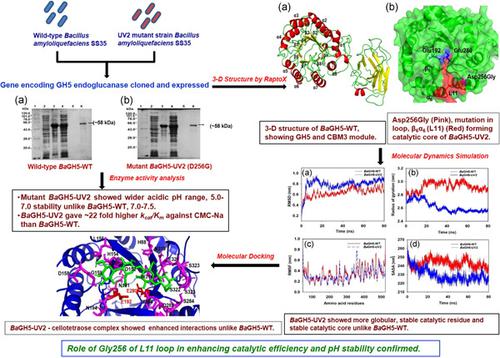
Wild‐type, BaGH5‐WT and mutant, BaGH5‐UV2 (aspartate residue mutated to glycine), endoglucanases belonging to glycoside hydrolase family 5 (GH5), from wild‐type, and UV2 mutant strain of Bacillus amyloliquefaciens SS35, respectively, were earlier cloned in pHTP0 cloning vector. In this study, genes encoding BaGH5‐WT or BaGH5‐UV2 were cloned into pET28a(+) expression‐vector and expressed in Escherichia coli BL‐21(DE3)pLysS cells. BaGH5‐UV2 showed 10‐fold (43.6 U/mg) higher specific activity against carboxymethylcellulose sodium salt (CMC‐Na), higher optimal temperature by 10°C at 65°C, and 22‐fold higher catalytic efficiency against CMC‐Na, than BaGH5‐WT. BaGH5‐UV2 showed stability in wider acidic pH range (5.0–7.0) unlike BaGH5‐WT in narrow basic pH range (7.0–7.5). BaGH5‐UV2 displayed a mutation, Asp256Gly in L11 loop, connecting β6‐sheet with α6‐helix, near active site toward the domain surface of (α/β)8‐TIM barrel fold. Molecular dynamics simulation studies showed more stable structure, accessibility of substrate for a catalytic site, and increased flexibility of loop L11 of BaGH5‐UV2 than the wild type, suggesting enhanced catalysis by BaGH5‐UV2. Molecular docking analysis displayed enhanced hydrogen bond interactions of cello‐oligosaccharides with BaGH5‐UV2, unlike BaGH5‐WT. Thus, Gly256 residue of loop L11 plays an important role in enhancing catalytic efficiency, and pH stability of GH5 endoglucanase. Therefore, these results help in protein engineering of GH5 endoglucanase for improved biochemical properties.
中文翻译:

甘氨酸 256 残基在提高解淀粉芽孢杆菌 SS35 家族 5 糖苷水解酶突变内切葡聚糖酶催化效率中的作用。
野生型、Ba GH5-WT 和突变体、Ba GH5-UV2(天冬氨酸残基突变为甘氨酸)、属于糖苷水解酶家族 5 (GH5) 的内切葡聚糖酶,分别来自野生型和解淀粉芽孢杆菌SS35 的UV2 突变株,早先被克隆到 pHTP0 克隆载体中。在这项研究中,编码Ba GH5-WT 或Ba GH5-UV2 的基因被克隆到 pET28a(+) 表达载体中,并在大肠杆菌BL-21(DE3)pLysS 细胞中表达。Ba GH5-UV2 对羧甲基纤维素钠盐 (CMC-Na) 的比活性提高了 10 倍 (43.6 U/mg),在 65°C 时的最适温度提高了 10°C,对 CMC-Na 的催化效率提高了 22 倍, 比Ba GH5-WT。Ba GH5-UV2 在较宽的酸性 pH 范围(5.0-7.0)中表现出稳定性,而Ba GH5-WT 在较窄的碱性 pH 范围(7.0-7.5)中表现出稳定性。Ba GH5-UV2 在 L11 环中显示出一个突变,Asp256Gly,将 β 6片层与 α 6螺旋连接,靠近活性位点,朝向 (α/β) 8 -TIM 桶状折叠的结构域表面。分子动力学模拟研究表明,与野生型相比,Ba GH5-UV2的环 L11 具有更稳定的结构、催化位点的可接近性和更高的灵活性,表明Ba GH5-UV2 的催化作用增强。分子对接分析显示纤维寡糖与Ba GH5-UV2,不同于Ba GH5-WT。因此,环 L11 的 Gly256 残基在提高催化效率和 GH5 内切葡聚糖酶的 pH 稳定性方面起着重要作用。因此,这些结果有助于 GH5 内切葡聚糖酶的蛋白质工程,以改善生化特性。
更新日期:2020-06-02
中文翻译:

甘氨酸 256 残基在提高解淀粉芽孢杆菌 SS35 家族 5 糖苷水解酶突变内切葡聚糖酶催化效率中的作用。
野生型、Ba GH5-WT 和突变体、Ba GH5-UV2(天冬氨酸残基突变为甘氨酸)、属于糖苷水解酶家族 5 (GH5) 的内切葡聚糖酶,分别来自野生型和解淀粉芽孢杆菌SS35 的UV2 突变株,早先被克隆到 pHTP0 克隆载体中。在这项研究中,编码Ba GH5-WT 或Ba GH5-UV2 的基因被克隆到 pET28a(+) 表达载体中,并在大肠杆菌BL-21(DE3)pLysS 细胞中表达。Ba GH5-UV2 对羧甲基纤维素钠盐 (CMC-Na) 的比活性提高了 10 倍 (43.6 U/mg),在 65°C 时的最适温度提高了 10°C,对 CMC-Na 的催化效率提高了 22 倍, 比Ba GH5-WT。Ba GH5-UV2 在较宽的酸性 pH 范围(5.0-7.0)中表现出稳定性,而Ba GH5-WT 在较窄的碱性 pH 范围(7.0-7.5)中表现出稳定性。Ba GH5-UV2 在 L11 环中显示出一个突变,Asp256Gly,将 β 6片层与 α 6螺旋连接,靠近活性位点,朝向 (α/β) 8 -TIM 桶状折叠的结构域表面。分子动力学模拟研究表明,与野生型相比,Ba GH5-UV2的环 L11 具有更稳定的结构、催化位点的可接近性和更高的灵活性,表明Ba GH5-UV2 的催化作用增强。分子对接分析显示纤维寡糖与Ba GH5-UV2,不同于Ba GH5-WT。因此,环 L11 的 Gly256 残基在提高催化效率和 GH5 内切葡聚糖酶的 pH 稳定性方面起着重要作用。因此,这些结果有助于 GH5 内切葡聚糖酶的蛋白质工程,以改善生化特性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号