当前位置:
X-MOL 学术
›
J. Chem. Thermodyn.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thermal properties of molybdenum hexacarbonyl: Kinetic and thermodynamic studies
The Journal of Chemical Thermodynamics ( IF 2.2 ) Pub Date : 2020-11-01 , DOI: 10.1016/j.jct.2020.106174 Faouzi Ayari , Emna Mannei , Esther Asedegbega-Nieto
The Journal of Chemical Thermodynamics ( IF 2.2 ) Pub Date : 2020-11-01 , DOI: 10.1016/j.jct.2020.106174 Faouzi Ayari , Emna Mannei , Esther Asedegbega-Nieto
|
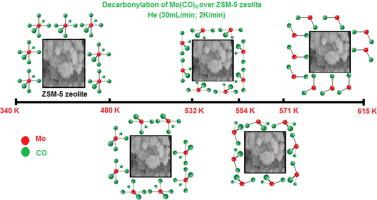
Abstract The thermal analysis of molybdenum hexacarbonyl has been performed under helium stream (2 K min−1) between room temperature and (1100 ± 2) K. According to mass spectrometry results, the organometallic sublimates between (330.4 ± 0.1) K and (339.7 ± 0.1) K, and the sublimation enthalpy, determined at two particular temperatures (ΔsubH(298.15 K) = (73.66 ± 0.02) kJ mol−1), is similar to the activation energy. As a matter of fact, the sublimation of Mo(CO)6(cr) follows a perfect-zero order reaction kinetic (Ea = (73.49 ± 0.10) kJ mol−1 and the pre-exponential factor is (8.33 ± 0.33) × 108 s−1). Furthermore, using Langmuir equation and thermogravimetry data, we were able to determine the vaporization constant (α) (=8.95 × 10−5 ± 1.45 × 10−7), the constant k (=80735 J0.5 mol−0.5 K−0.5) as well as ΔsubH (298.15 K) (equal to (75.91 ± 0.37) kJ mol−1). In the presence of ZSM-5 zeolite, the temperatures of Mo(CO)6(cr) decarbonylation, Ea and ΔsubH values were succinctly determined. Interestingly, the loss of the first CO ligand in Mo(CO)6(cr) occurred between (480 ± 0.1) K and (532 ± 0.1) K.
中文翻译:

六羰基钼的热性能:动力学和热力学研究
摘要 六羰基钼在氦气流(2 K min-1)下在室温和(1100±2)K之间进行了热分析。根据质谱结果,有机金属在(330.4±0.1)K和(339.7)之间升华± 0.1) K 和在两个特定温度下确定的升华焓 (ΔsubH(298.15 K) = (73.66 ± 0.02) kJ mol-1),与活化能相似。事实上,Mo(CO)6(cr)的升华遵循完全零级反应动力学(Ea = (73.49 ± 0.10) kJ mol−1,指前因子为(8.33 ± 0.33) × 108 秒-1)。此外,使用朗缪尔方程和热重数据,我们能够确定蒸发常数 (α) (=8.95 × 10−5 ± 1.45 × 10−7),常数 k (=80735 J0.5 mol−0.5 K−0.5 ) 以及 ΔsubH (298.15 K)(等于 (75.91 ± 0. 37) kJ mol−1)。在ZSM-5沸石的存在下,Mo(CO)6(cr)脱羰的温度、Ea和ΔsubH值被简洁地确定。有趣的是,Mo(CO)6(cr) 中第一个 CO 配体的丢失发生在 (480 ± 0.1) K 和 (532 ± 0.1) K 之间。
更新日期:2020-11-01
中文翻译:

六羰基钼的热性能:动力学和热力学研究
摘要 六羰基钼在氦气流(2 K min-1)下在室温和(1100±2)K之间进行了热分析。根据质谱结果,有机金属在(330.4±0.1)K和(339.7)之间升华± 0.1) K 和在两个特定温度下确定的升华焓 (ΔsubH(298.15 K) = (73.66 ± 0.02) kJ mol-1),与活化能相似。事实上,Mo(CO)6(cr)的升华遵循完全零级反应动力学(Ea = (73.49 ± 0.10) kJ mol−1,指前因子为(8.33 ± 0.33) × 108 秒-1)。此外,使用朗缪尔方程和热重数据,我们能够确定蒸发常数 (α) (=8.95 × 10−5 ± 1.45 × 10−7),常数 k (=80735 J0.5 mol−0.5 K−0.5 ) 以及 ΔsubH (298.15 K)(等于 (75.91 ± 0. 37) kJ mol−1)。在ZSM-5沸石的存在下,Mo(CO)6(cr)脱羰的温度、Ea和ΔsubH值被简洁地确定。有趣的是,Mo(CO)6(cr) 中第一个 CO 配体的丢失发生在 (480 ± 0.1) K 和 (532 ± 0.1) K 之间。











































 京公网安备 11010802027423号
京公网安备 11010802027423号