Structure ( IF 4.4 ) Pub Date : 2020-06-02 , DOI: 10.1016/j.str.2020.04.021 Marc A Dämgen 1 , Afroditi Maria Zaki 1 , Philip C Biggin 1
|
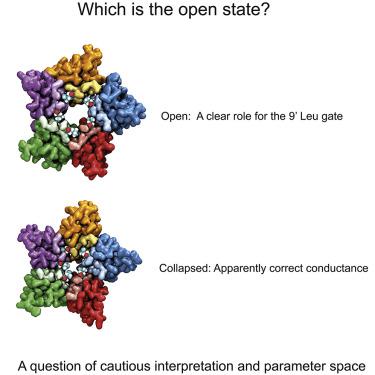
Recently, we reported the simulation of a stable open state of the glycine receptor. Central to the stability of the simulations was the behavior of the highly conserved leucine residues at the 9′ gate, which were found to rotate into adjacent pockets, thus providing a structural rationale for decades of biochemical observations. In contrast, a previously reported model from Cerdan et al. (2018) resembled a more collapsed state. However, in support of their model, they draw attention to the agreement between calculated and experimental conductance measurements and argue that our model tends to overestimate ion flow. Here, we argue that there are many pitfalls with this approach and that the apparent agreement most likely reflects a fortuitous cancellation of errors. The computed values are highly sensitive to very small changes in model parameters. It is also likely that polarization effects will be very significant, and these have not yet been considered.
中文翻译:

评论“关于甘氨酸受体中开放通道结构的功能注释”。
最近,我们报道了甘氨酸受体稳定开放状态的模拟。模拟稳定性的核心是 9' 门处高度保守的亮氨酸残基的行为,发现这些残基会旋转到相邻的口袋中,从而为数十年的生化观察提供了结构依据。相比之下,Cerdan 等人先前报道的模型。(2018)类似于一个更加崩溃的状态。然而,为了支持他们的模型,他们提请注意计算和实验电导测量之间的一致性,并认为我们的模型倾向于高估离子流。在这里,我们认为这种方法存在许多缺陷,并且明显的一致性很可能反映了偶然消除了错误。计算值对模型参数的微小变化高度敏感。











































 京公网安备 11010802027423号
京公网安备 11010802027423号