Structure ( IF 4.4 ) Pub Date : 2020-06-02 , DOI: 10.1016/j.str.2020.05.003 Adrien Henri Cerdan 1 , Marco Cecchini 2
|
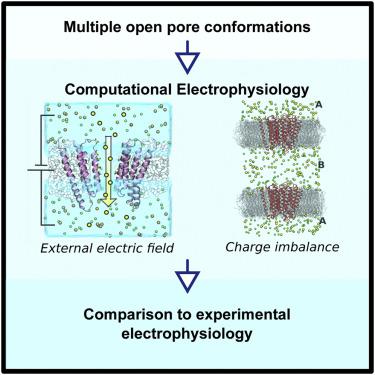
The glycine receptor (GlyR) is by far the best-characterized pentameric ligand-gated ion channel, with several high-resolution structures from X-ray crystallography, cryoelectron microscopy (cryo-EM), and modeling. Nonetheless, the significance of the currently available open-pore conformations is debated due to their diversity in the pore geometry. Here, we discuss the physiological significance of existing models of the GlyR active state based on conductance and selectivity measurements by computational electrophysiology. The results support the conclusion that the original cryo-EM reconstruction of the active state obtained in detergents as well as its subsequent refinement by molecular dynamics simulations are likely to be non-physiological as they feature artificially dilated ion pores. In addition, the calculations indicate that a physiologically relevant open pore should be constricted within a radius of 2.5 and 2.8 Å, which is consistent with previous modeling, electrophysiology measurements, and the most recent cryo-EM structures obtained in a native lipid membrane environment.
中文翻译:

甘氨酸受体中开放通道结构的功能注释。
甘氨酸受体(GlyR)是迄今为止表征最完善的五聚体配体门控离子通道,具有X射线晶体学,低温电子显微镜(cryo-EM)和建模等多种高分辨率结构。然而,由于孔几何形状的多样性,目前可用的开孔构象的重要性尚有争议。在这里,我们讨论基于电导率和选择性电生理测量的GlyR活跃状态的现有模型的生理意义。结果支持这样的结论,即在洗涤剂中获得的原始冷冻-EM活性状态的重建及其随后通过分子动力学模拟进行的提纯很可能是非生理性的,因为它们具有人工膨胀的离子孔。此外,











































 京公网安备 11010802027423号
京公网安备 11010802027423号