Journal of Proteomics ( IF 2.8 ) Pub Date : 2020-06-02 , DOI: 10.1016/j.jprot.2020.103850 Bindu Nanduri 1 , Cathy R Gresham 2 , Winnie W Hui 3 , Mark Ou 3 , Richard H Bailey 4 , Mariola J Edelmann 3
|
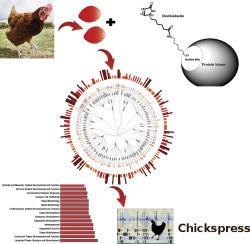
Phosphorylation is a post-translational protein modification regulating most known cellular processes. While protein kinases constitute a large family of highly conserved enzymes, identification of active kinases is challenging due to a low abundance of some of these signaling molecules. Although chicken is the first agricultural animal to have a sequenced genome, annotation of the kinome, i.e., a complement of all protein kinases in the genome is limited. We used chemical probes consisting of ATP and ADP derivatives binding to specific lysine (Lys) residues within the ATP-binding pocket of kinases, combined with proteomics, to identify 267 peptides labeled with the ATP and ADP acyl derivatives and 188 corresponding chicken kinases in chicken spleen and liver. Our description of active chicken kinases and ATP binding sites will support future studies focused on identifying the role of this important class of enzymes in chicken health and disease.
Significance
Advances made in understanding chicken enzymes are critical for the improved knowledge of the regulatory pathways controlling physiological processes in chicken. Since protein phosphorylation controls multiple aspects of cell fate, it is often linked to pathological conditions, and understanding of the kinase expression in chicken is essential for future therapeutic approaches. We coupled proteomics and labeling with active-site probes binding to Lys residues within the ATP-binding pocket of kinases to identify 188 kinases and corresponding 267 peptides labeled with the ATP and ADP acyl derivatives in chicken spleen and liver. Results of the present study describing catalytically active kinases is a starting point for chemoproteomic-based interrogation of kinases in chicken exposed to different conditions. Kinases identified in this study are available through the Chickspress genome browser that has previously published mRNA, miRNA, and shotgun proteomics data.
中文翻译:

化学蛋白质组学鉴定的鸡催化活性肝和脾激酶图集。
磷酸化是调节大多数已知细胞过程的翻译后蛋白修饰。尽管蛋白激酶构成了一个高度保守的酶家族,但由于其中一些信号分子的丰度低,因此鉴定活性激酶是一项挑战。尽管鸡是第一个具有序列化基因组的农用动物,但是对基因组的注释,即基因组中所有蛋白激酶的互补物,是有限的。我们使用了由结合在激酶的ATP结合口袋中的特定赖氨酸(Lys)残基的ATP和ADP衍生物组成的化学探针,结合蛋白质组学,来鉴定267种标有ATP和ADP酰基衍生物标记的肽和188种相应的鸡激酶脾肝。
意义
了解鸡肉酶方面的进展对于提高对鸡肉生理过程的调控途径的认识至关重要。由于蛋白质的磷酸化控制着细胞命运的多个方面,因此它通常与病理状况有关,了解鸡中激酶的表达对于未来的治疗方法至关重要。我们将蛋白质组学和标记与结合到激酶的ATP结合口袋中的Lys残基的活性位点探针结合起来,以鉴定188种激酶和相应的267种肽,这些肽标记有鸡脾脏和肝脏中的ATP和ADP酰基衍生物。描述催化活性激酶的本研究结果是暴露于不同条件的鸡肉中基于化学计量学的激酶询问的起点。











































 京公网安备 11010802027423号
京公网安备 11010802027423号