Journal of Biotechnology ( IF 4.1 ) Pub Date : 2020-06-02 , DOI: 10.1016/j.jbiotec.2020.05.011 J Koop 1 , J Merz 2 , R Wilmshöfer 1 , R Winter 3 , G Schembecker 1
|
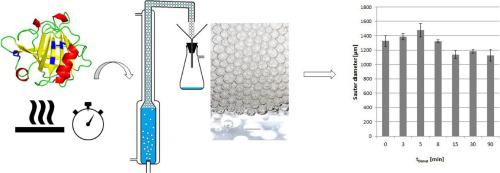
Surface activity is an intrinsic protein feature, leading to the capability of aqueous protein solutions to form foam. This feature provides opportunities for downstream processing, such as usage of foam fractionation for purification. In order to investigate the impact of the surface activity on the performance of the foam fractionation process, protein solutions with different surface activity were produced by different thermal denaturation of aqueous β-lactoglobulin solutions. The effectiveness of the denaturation procedure was verified with circular dichroic spectroscopy, and the impact on surface activity was determined via dynamic surface tension measurement. The increased surface activity resulted in higher foamate flow rates. Furthermore, the effects could be correlated with secondary structure changes and with the dynamic surface pressure. The new result of this study is that the effect of the denaturation of a protein on foam fractionation depends on the protein concentration. At the lower feed concentration, effects became visible, which could not be observed at the higher concentration.
中文翻译:

稀释的β-乳球蛋白溶液中热诱导结构变化对其表面活性和泡沫分离行为的影响。
表面活性是蛋白质的固有特征,从而导致蛋白质水溶液形成泡沫的能力。此功能为下游处理提供了机会,例如使用泡沫分离进行纯化。为了研究表面活性对泡沫分离过程性能的影响,通过β-乳球蛋白水溶液的不同热变性来制备具有不同表面活性的蛋白质溶液。通过圆二色光谱验证了变性程序的有效性,并通过动态表面张力测量确定了对表面活性的影响。增加的表面活性导致较高的泡沫流速。此外,这些影响可能与二级结构的变化以及动态表面压力有关。这项研究的新结果是蛋白质变性对泡沫分离的影响取决于蛋白质浓度。在较低的进料浓度下,效果可见,而在较高的进料浓度下则无法观察到。











































 京公网安备 11010802027423号
京公网安备 11010802027423号