Cell ( IF 45.5 ) Pub Date : 2020-06-02 , DOI: 10.1016/j.cell.2020.05.012 Sara Zaccara 1 , Samie R Jaffrey 1
|
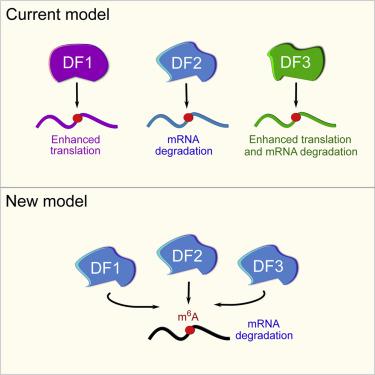
N6-methyladenosine (m6A) is the most abundant mRNA nucleotide modification and regulates critical aspects of cellular physiology and differentiation. m6A is thought to mediate its effects through a complex network of interactions between different m6A sites and three functionally distinct cytoplasmic YTHDF m6A-binding proteins (DF1, DF2, and DF3). In contrast to the prevailing model, we show that DF proteins bind the same m6A-modified mRNAs rather than different mRNAs. Furthermore, we find that DF proteins do not induce translation in HeLa cells. Instead, the DF paralogs act redundantly to mediate mRNA degradation and cellular differentiation. The ability of DF proteins to regulate stability and differentiation becomes evident only when all three DF paralogs are depleted simultaneously. Our study reveals a unified model of m6A function in which all m6A-modified mRNAs are subjected to the combined action of YTHDF proteins in proportion to the number of m6A sites.
中文翻译:

YTHDF 蛋白调节 m6A 修饰 mRNA 功能的统一模型。
N 6 -甲基腺苷 (m 6 A) 是最丰富的 mRNA 核苷酸修饰,调节细胞生理学和分化的关键方面。 m 6 A 被认为通过不同 m 6 A 位点和三个功能不同的细胞质 YTHDF m 6 A 结合蛋白(DF1、DF2 和 DF3)之间相互作用的复杂网络来介导其作用。与流行的模型相反,我们发现 DF 蛋白结合相同的 m 6 A 修饰的 mRNA,而不是不同的 mRNA。此外,我们发现 DF 蛋白不会诱导 HeLa 细胞中的翻译。相反,DF 旁系同源物冗余地发挥作用来介导 mRNA 降解和细胞分化。仅当所有三种 DF 旁系同源物同时耗尽时,DF 蛋白调节稳定性和分化的能力才变得明显。我们的研究揭示了 m 6 A 功能的统一模型,其中所有 m 6 A 修饰的 mRNA 均受到 YTHDF 蛋白的联合作用,与 m 6 A 位点的数量成比例。











































 京公网安备 11010802027423号
京公网安备 11010802027423号