当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Recent advances in the oxime-participating synthesis of isoxazolines.
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2020-06-01 , DOI: 10.1039/d0ob00963f Jianhua Liao 1 , Lu Ouyang 2 , Qi Jin 2 , Jian Zhang 2 , Renshi Luo 2
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2020-06-01 , DOI: 10.1039/d0ob00963f Jianhua Liao 1 , Lu Ouyang 2 , Qi Jin 2 , Jian Zhang 2 , Renshi Luo 2
Affiliation
|
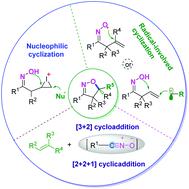
Isoxazoline compounds are used as important intermediates for the synthesis of organic molecules, which are widely used in the chemical and life science industries. Oxime-participating cyclization has emerged as an efficient strategy for the construction of isoxazolines. This review is devoted to highlighting the main achievements (since 2010) in the development of methodologies for the synthesis of isoxazolines. According to the reaction mechanism, the oxime-participating synthesis of isoxazolines can be mainly classified into four reaction types: iminoxyl radical-initiated intramolecular cyclization, intermolecular radical addition-initiated cyclization, intramolecular nucleophilic cyclization, and [3 + 2] cycloaddition. Meanwhile, miscellaneous examples are also illustrated, such as [2 + 2 + 1] cycloaddition. Representative reactions will be discussed for each of the highlighted synthetic strategies. In addition, the enantioselective synthesis of isoxazolines is also illustrated in this review.
中文翻译:

参与肟的异恶唑啉合成的最新进展。
异恶唑啉化合物用作有机分子合成的重要中间体,已广泛用于化学和生命科学行业。参与肟的环化反应已成为构建异恶唑啉的有效策略。这篇综述致力于突出自2010年以来在合成异恶唑啉方法学方面的主要成就。根据反应机理,参与肟的异恶唑啉的合成可主要分为四种反应类型:亚氨基氧基引发的分子内环化,分子间自由基加成引发的环化,分子内亲核环化和[3 + 2]环加成。同时,还说明了其他示例,例如[2 + 2 +1]环加成。对于每种突出的合成策略,将讨论代表性反应。此外,本综述还说明了异恶唑啉的对映选择性合成。
更新日期:2020-07-01
中文翻译:

参与肟的异恶唑啉合成的最新进展。
异恶唑啉化合物用作有机分子合成的重要中间体,已广泛用于化学和生命科学行业。参与肟的环化反应已成为构建异恶唑啉的有效策略。这篇综述致力于突出自2010年以来在合成异恶唑啉方法学方面的主要成就。根据反应机理,参与肟的异恶唑啉的合成可主要分为四种反应类型:亚氨基氧基引发的分子内环化,分子间自由基加成引发的环化,分子内亲核环化和[3 + 2]环加成。同时,还说明了其他示例,例如[2 + 2 +1]环加成。对于每种突出的合成策略,将讨论代表性反应。此外,本综述还说明了异恶唑啉的对映选择性合成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号