当前位置:
X-MOL 学术
›
Dalton Trans.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Diversity and uniformity in anion-π complexes of thiocyanate with aromatic, olefinic and quinoidal π-acceptors.
Dalton Transactions ( IF 3.5 ) Pub Date : 2020-06-01 , DOI: 10.1039/d0dt01654c Joshua Wilson 1 , Tristan Maxson , Isabelle Wright , Matthias Zeller , Sergiy V Rosokha
Dalton Transactions ( IF 3.5 ) Pub Date : 2020-06-01 , DOI: 10.1039/d0dt01654c Joshua Wilson 1 , Tristan Maxson , Isabelle Wright , Matthias Zeller , Sergiy V Rosokha
Affiliation
|
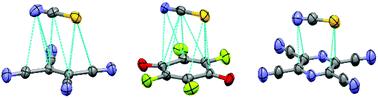
Despite the progress in the study of anion–π interactions, there are still inconsistencies in the use of this term and the experimental data about factors affecting the strength of such bonding are limited. To shed light on these issues, we explored supramolecular associations between NCS− anions and a series of aromatic, olefinic or quinoidal π-acceptors. Combined experimental and computational studies revealed that all these complexes were formed by an attraction of the anion to the face of the π-system, and the arrangements of thiocyanate followed the areas of the most positive potentials on the surfaces of the π-acceptors. The stabilities of the complexes increased with the π-acceptor strength (reflected by their reduction potentials), and were essentially independent of the magnitudes of the maximum electrostatic potentials on their surfaces. The complexes showed intense absorption bands in the UV-Vis range, and the energies of these bands were correlated with the difference of the redox potentials of the anions and π-acceptors. Such features, as well as results of atoms-in-molecules and non-covalent index analyses suggested that besides electrostatics, molecular orbital interactions play a substantial role in the formation of these complexes. The unified trends in variations of the characteristics of the complexes between thiocyanate and a variety of π-acceptors point to their common nature. To embrace diversity and uniformity of the anion–π associates, we suggest (following the halogen bond's definition) that anion–π bonding occurs when there is evidence of a net attraction between the anions and the face of the electrophilic π-system.
中文翻译:

硫氰酸盐与芳香族,烯属和醌型π受体的阴离子π配合物的多样性和均匀性。
尽管在阴离子-π相互作用的研究方面取得了进展,但该术语的使用仍然存在不一致之处,并且有关影响这种键合强度的因素的实验数据仍然有限。为了阐明这些问题,我们探讨了NCS之间的超分子关联-阴离子和一系列芳香族,烯属或喹啉π受体。组合的实验和计算研究表明,所有这些络合物都是由阴离子对π系统表面的吸引所形成的,硫氰酸盐的排列遵循π受体表面上最具正电势的区域。配合物的稳定性随π受体强度的增加(由其还原电位反映),并且基本上与它们表面上最大静电电位的大小无关。配合物在UV-Vis范围内显示出很强的吸收带,并且这些带的能量与阴离子和π受体的氧化还原电位的差异相关。这样的功能 以及分子中原子和非共价指数分析的结果表明,除了静电以外,分子轨道相互作用在这些络合物的形成中也起着重要作用。硫氰酸盐和各种π受体之间的配合物的特性变化的统一趋势表明了它们的共同性质。为了包含阴离子-π缔合体的多样性和均匀性,我们建议(按照卤素键的定义),当有证据表明阴离子与亲电π系统的表面之间存在净吸引时,就会发生阴离子-π键。硫氰酸盐和各种π受体之间的配合物的特性变化的统一趋势表明了它们的共同性质。为了包含阴离子-π缔合体的多样性和均匀性,我们建议(按照卤素键的定义),当有证据表明阴离子与亲电π系统的表面之间存在净吸引时,就会发生阴离子-π键。硫氰酸盐和各种π受体之间的配合物的特性变化的统一趋势表明了它们的共同性质。为了包含阴离子-π缔合体的多样性和均匀性,我们建议(按照卤素键的定义),当有证据表明阴离子与亲电π系统的表面之间存在净吸引时,就会发生阴离子-π键。
更新日期:2020-06-29
中文翻译:

硫氰酸盐与芳香族,烯属和醌型π受体的阴离子π配合物的多样性和均匀性。
尽管在阴离子-π相互作用的研究方面取得了进展,但该术语的使用仍然存在不一致之处,并且有关影响这种键合强度的因素的实验数据仍然有限。为了阐明这些问题,我们探讨了NCS之间的超分子关联-阴离子和一系列芳香族,烯属或喹啉π受体。组合的实验和计算研究表明,所有这些络合物都是由阴离子对π系统表面的吸引所形成的,硫氰酸盐的排列遵循π受体表面上最具正电势的区域。配合物的稳定性随π受体强度的增加(由其还原电位反映),并且基本上与它们表面上最大静电电位的大小无关。配合物在UV-Vis范围内显示出很强的吸收带,并且这些带的能量与阴离子和π受体的氧化还原电位的差异相关。这样的功能 以及分子中原子和非共价指数分析的结果表明,除了静电以外,分子轨道相互作用在这些络合物的形成中也起着重要作用。硫氰酸盐和各种π受体之间的配合物的特性变化的统一趋势表明了它们的共同性质。为了包含阴离子-π缔合体的多样性和均匀性,我们建议(按照卤素键的定义),当有证据表明阴离子与亲电π系统的表面之间存在净吸引时,就会发生阴离子-π键。硫氰酸盐和各种π受体之间的配合物的特性变化的统一趋势表明了它们的共同性质。为了包含阴离子-π缔合体的多样性和均匀性,我们建议(按照卤素键的定义),当有证据表明阴离子与亲电π系统的表面之间存在净吸引时,就会发生阴离子-π键。硫氰酸盐和各种π受体之间的配合物的特性变化的统一趋势表明了它们的共同性质。为了包含阴离子-π缔合体的多样性和均匀性,我们建议(按照卤素键的定义),当有证据表明阴离子与亲电π系统的表面之间存在净吸引时,就会发生阴离子-π键。











































 京公网安备 11010802027423号
京公网安备 11010802027423号