当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
PtRu/Zn3Ce1Ox catalysts with Lewis acid–base pairs show synergistic performances for the conversion of glycerol in the absence of externally added H2
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2020-06-02 , DOI: 10.1039/c9cy02465d Guangyu Zhang 1, 2, 3, 4, 5 , Xin Jin 1, 2, 3, 4, 5 , Quanxing Zhang 1, 2, 3, 4, 5 , Yinlei Cheng 1, 2, 3, 4, 5 , Xiaobo Chen 1, 2, 3, 4, 5 , Yibin Liu 1, 2, 3, 4, 5 , Xiang Feng 1, 2, 3, 4, 5 , Chaohe Yang 1, 2, 3, 4, 5
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2020-06-02 , DOI: 10.1039/c9cy02465d Guangyu Zhang 1, 2, 3, 4, 5 , Xin Jin 1, 2, 3, 4, 5 , Quanxing Zhang 1, 2, 3, 4, 5 , Yinlei Cheng 1, 2, 3, 4, 5 , Xiaobo Chen 1, 2, 3, 4, 5 , Yibin Liu 1, 2, 3, 4, 5 , Xiang Feng 1, 2, 3, 4, 5 , Chaohe Yang 1, 2, 3, 4, 5
Affiliation
|
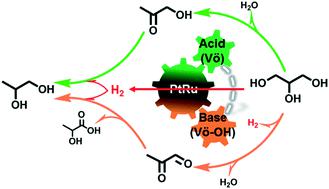
Previous studies revealed that tandem dehydration, dehydrogenation and hydrogenation, promoted by metal–support interfacial catalysis, play a critical role in determining the intrinsic kinetics of transfer hydrogenolysis of bio-oxygenates. However, the synergism of metal and acid–base pairs for tunable C–H, O–H and C–O cleavage and metal–H strength is under debate in this area. Herein, we present a series of bimetallic PtRu/Zn3Ce1-LDO catalysts, with abundant Lewis acid–base pairs and heterojunction structures at the metal–support interface, exhibiting enhanced performances of transfer hydrogenolysis of glycerol. The key finding is that the incorporation of ZnO unexpectedly induced the formation of large amounts of oxygen vacant sites and surface hydroxyl sites on the CeOx support, leading to abundant Lewis acid–base pairs. The strong electron coupling effect of PtRu and Lewis acid–base pairs results in weakened PtRu–H bonding, facilitating a hydrogen transfer reaction. Synergism between enhanced Lewis acid–base pairs and weaker PtRu–H hydride bonding is critical for remarkable catalytic activity (TOF: 526 h−1) and selectivity towards glycols (70.8%), and results in one of the best performances in the current literature. Moreover, the influence of hydrogen donors, reaction temperature and time on conversion and product distribution was further investigated in detail. An alternative reaction pathway for the transfer hydrogenolysis of glycerol over the proposed acid–base pair catalysts was proposed and validated with experimental data. The outcome of this work will provide new insights into the rational design of efficient catalytic materials for energy and environmental applications.
中文翻译:

具有路易斯酸碱对的PtRu / Zn3Ce1Ox催化剂在没有外部添加H2的情况下显示出甘油转化的协同性能
先前的研究表明,由金属支持的界面催化促进的串联脱水,脱氢和氢化在决定生物加氧物转移氢解的内在动力学方面起着至关重要的作用。然而,在这一领域,金属和酸碱对可调节的CH,OH和CH裂解以及金属H强度的协同作用尚在争论中。在这里,我们介绍了一系列双金属PtRu / Zn 3 Ce 1 -LDO催化剂,在金属-载体界面上具有丰富的路易斯酸碱对和异质结结构,表现出增强的转移式甘油氢解性能。关键发现是ZnO的掺入意外地诱导了CeO上大量氧空位和表面羟基的形成x的支持,导致大量的路易斯酸碱对。PtRu和路易斯酸碱对的强电子耦合作用导致PtRu-H键减弱,从而促进了氢转移反应。增强的路易斯酸-碱对和弱的PtRu-H氢化物键之间的协同作用对于出色的催化活性至关重要(TOF:526 h -1)和对乙二醇的选择性(70.8%),是目前文献中表现最好的一种。此外,进一步详细研究了氢供体,反应温度和时间对转化率和产物分布的影响。提出了在提议的酸碱对催化剂上进行甘油转移氢解反应的另一种反应途径,并通过实验数据进行了验证。这项工作的结果将为合理设计用于能源和环境应用的高效催化材料提供新的见解。
更新日期:2020-07-06
中文翻译:

具有路易斯酸碱对的PtRu / Zn3Ce1Ox催化剂在没有外部添加H2的情况下显示出甘油转化的协同性能
先前的研究表明,由金属支持的界面催化促进的串联脱水,脱氢和氢化在决定生物加氧物转移氢解的内在动力学方面起着至关重要的作用。然而,在这一领域,金属和酸碱对可调节的CH,OH和CH裂解以及金属H强度的协同作用尚在争论中。在这里,我们介绍了一系列双金属PtRu / Zn 3 Ce 1 -LDO催化剂,在金属-载体界面上具有丰富的路易斯酸碱对和异质结结构,表现出增强的转移式甘油氢解性能。关键发现是ZnO的掺入意外地诱导了CeO上大量氧空位和表面羟基的形成x的支持,导致大量的路易斯酸碱对。PtRu和路易斯酸碱对的强电子耦合作用导致PtRu-H键减弱,从而促进了氢转移反应。增强的路易斯酸-碱对和弱的PtRu-H氢化物键之间的协同作用对于出色的催化活性至关重要(TOF:526 h -1)和对乙二醇的选择性(70.8%),是目前文献中表现最好的一种。此外,进一步详细研究了氢供体,反应温度和时间对转化率和产物分布的影响。提出了在提议的酸碱对催化剂上进行甘油转移氢解反应的另一种反应途径,并通过实验数据进行了验证。这项工作的结果将为合理设计用于能源和环境应用的高效催化材料提供新的见解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号