Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Functionalized Mesoporous Silicas Direct Structural Polymorphism of Amyloid-β Fibrils.
Langmuir ( IF 3.7 ) Pub Date : 2020-06-01 , DOI: 10.1021/acs.langmuir.0c00827 Michael J Lucas 1 , Henry S Pan 1 , Eric J Verbeke 2 , Lauren J Webb 3 , David W Taylor 2, 4, 5, 6 , Benjamin K Keitz 1
Langmuir ( IF 3.7 ) Pub Date : 2020-06-01 , DOI: 10.1021/acs.langmuir.0c00827 Michael J Lucas 1 , Henry S Pan 1 , Eric J Verbeke 2 , Lauren J Webb 3 , David W Taylor 2, 4, 5, 6 , Benjamin K Keitz 1
Affiliation
|
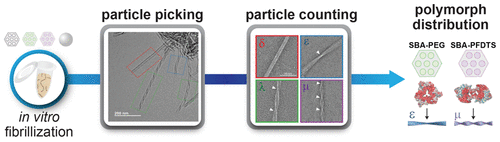
The aggregation of amyloid-β (Aβ) is associated with the onset of Alzheimer’s disease (AD) and involves a complex kinetic pathway as monomers self-assemble into fibrils. A central feature of amyloid fibrils is the existence of multiple structural polymorphs, which complicates the development of disease-relevant structure–function relationships. Developing these relationships requires new methods to control fibril structure. In this work, we evaluated the effect that mesoporous silicas (SBA-15) functionalized with hydrophobic (SBA-PFDTS) and hydrophilic groups (SBA-PEG) have on the aggregation kinetics and resulting structure of Aβ1–40 fibrils. The hydrophilic SBA-PEG had little effect on amyloid kinetics, while as-synthesized and hydrophobic SBA-PFDTS accelerated aggregation kinetics. Subsequently, we quantified the relative population of fibril structures formed in the presence of each material using electron microscopy. Fibrils formed from Aβ1–40 exposed to SBA-PEG were structurally similar to control fibrils. In contrast, Aβ1–40 incubated with SBA-15 or SBA-PFDTS formed fibrils with shorter crossover distances that were more structurally representative of fibrils found in AD patient derived samples. Overall, our results suggest that mesoporous silicas and other exogenous materials are promising scaffolds for the de novo production of specific fibril polymorphs of Aβ1–40 and other amyloidogenic proteins.
中文翻译:

淀粉样β-原纤维的功能化介孔二氧化硅直接结构多态性。
淀粉样蛋白-β(Aβ)的聚集与阿尔茨海默氏病(AD)的发作有关,并且由于单体自组装成原纤维而涉及复杂的动力学路径。淀粉样蛋白原纤维的主要特征是存在多种结构多态性,这使与疾病相关的结构-功能关系的发展复杂化。建立这些关系需要新的方法来控制原纤维结构。在这项工作中,我们评估了用疏水性(SBA-PFDTS)和亲水性基团(SBA-PEG)官能化的中孔二氧化硅(SBA-15)对聚集动力学和所得Aβ1–40的结构的影响原纤维。亲水性SBA-PEG对淀粉样蛋白动力学影响很小,而合成后的疏水性SBA-PFDTS则加速了聚集动力学。随后,我们使用电子显微镜对在每种材料存在下形成的原纤维结构的相对种群进行了定量。由暴露于SBA-PEG的Aβ1–40形成的原纤维与对照原纤维在结构上相似。相反,Aβ 1-40孵育SBA-15或SBA-PFDTS具有较短距离交叉那个更结构代表在AD患者中发现原纤维的来源的样品形成原纤维。总体而言,我们的结果表明,介孔二氧化硅和其他外源材料是从头生产Aβ1–40特定原纤维多晶型物的有前途的支架 和其他淀粉样蛋白。
更新日期:2020-07-07
中文翻译:

淀粉样β-原纤维的功能化介孔二氧化硅直接结构多态性。
淀粉样蛋白-β(Aβ)的聚集与阿尔茨海默氏病(AD)的发作有关,并且由于单体自组装成原纤维而涉及复杂的动力学路径。淀粉样蛋白原纤维的主要特征是存在多种结构多态性,这使与疾病相关的结构-功能关系的发展复杂化。建立这些关系需要新的方法来控制原纤维结构。在这项工作中,我们评估了用疏水性(SBA-PFDTS)和亲水性基团(SBA-PEG)官能化的中孔二氧化硅(SBA-15)对聚集动力学和所得Aβ1–40的结构的影响原纤维。亲水性SBA-PEG对淀粉样蛋白动力学影响很小,而合成后的疏水性SBA-PFDTS则加速了聚集动力学。随后,我们使用电子显微镜对在每种材料存在下形成的原纤维结构的相对种群进行了定量。由暴露于SBA-PEG的Aβ1–40形成的原纤维与对照原纤维在结构上相似。相反,Aβ 1-40孵育SBA-15或SBA-PFDTS具有较短距离交叉那个更结构代表在AD患者中发现原纤维的来源的样品形成原纤维。总体而言,我们的结果表明,介孔二氧化硅和其他外源材料是从头生产Aβ1–40特定原纤维多晶型物的有前途的支架 和其他淀粉样蛋白。









































 京公网安备 11010802027423号
京公网安备 11010802027423号