Molecular Therapy - Methods & Clinical Development ( IF 4.6 ) Pub Date : 2020-06-01 , DOI: 10.1016/j.omtm.2020.05.028 Josse A Depla 1, 2 , Marina Sogorb-Gonzalez 1, 3 , Lance A Mulder 2 , Vivi M Heine 4, 5 , Pavlina Konstantinova 1 , Sander J van Deventer 1, 3 , Katja C Wolthers 2 , Dasja Pajkrt 6 , Adithya Sridhar 2 , Melvin M Evers 1
|
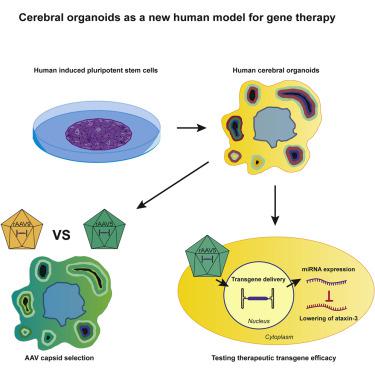
The development of gene therapies for central nervous system disorders is challenging because it is difficult to translate preclinical data from current in vitro and in vivo models to the clinic. Therefore, we developed induced pluripotent stem cell (iPSC)-derived cerebral organoids as a model for recombinant adeno-associated virus (rAAV) capsid selection and for testing efficacy of AAV-based gene therapy in a human context. Cerebral organoids are physiological 3D structures that better recapitulate the human brain compared with 2D cell lines. To validate the model, we compared the transduction efficiency and distribution of two commonly used AAV serotypes (rAAV5 and rAAV9). In cerebral organoids, transduction with rAAV5 led to higher levels of vector DNA, transgenic mRNA, and protein expression as compared with rAAV9. The superior transduction of rAAV5 was replicated in iPSC-derived neuronal cells. Furthermore, rAAV5-mediated delivery of a human sequence-specific engineered microRNA to cerebral organoids led to a lower expression of its target ataxin-3. Our studies provide a new tool for selecting and deselecting AAV serotypes, and for demonstrating therapeutic efficacy of transgenes in a human context. Implementing cerebral organoids during gene therapy development could reduce the usage of animal models and improve translation to the clinic.
中文翻译:

脑型器官:AAV衣壳选择和脑中治疗性转基因功效的人体模型。
对于中枢神经系统疾病的基因治疗的开发是具有挑战性的,因为它是难以转化从当前的临床前数据在体外和体内模型到诊所。因此,我们开发了诱导多能干细胞(iPSC)衍生的脑类器官,作为重组腺相关病毒(rAAV)衣壳选择和在人类环境中测试基于AAV的基因治疗功效的模型。脑类器官是3D生理结构,与2D细胞系相比,可以更好地重现人脑。为了验证模型,我们比较了两种常用的AAV血清型(rAAV5和rAAV9)的转导效率和分布。在脑类器官中,与rAAV9相比,rAAV5转导导致更高水平的载体DNA,转基因mRNA和蛋白质表达。在iPSC衍生的神经元细胞中复制了rAAV5的卓越转导。此外,rAAV5介导的人类序列特异性工程化microRNA向脑器官的传递导致其靶标抗生物素蛋白3的较低表达。我们的研究提供了一种选择和取消选择AAV血清型,并证明在人类环境中转基因的治疗功效的新工具。在基因治疗开发过程中实施脑类器官可减少动物模型的使用并改善临床翻译。









































 京公网安备 11010802027423号
京公网安备 11010802027423号