Molecular Therapy - Methods & Clinical Development ( IF 4.6 ) Pub Date : 2020-06-01 , DOI: 10.1016/j.omtm.2020.05.030 Vesa Anttila 1 , Antti Saraste 1, 2 , Juhani Knuuti 2 , Pekka Jaakkola 3 , Marja Hedman 3 , Sara Svedlund 4, 5 , Maria Lagerström-Fermér 6 , Magnus Kjaer 7 , Anders Jeppsson 5, 8 , Li-Ming Gan 5, 6, 9
|
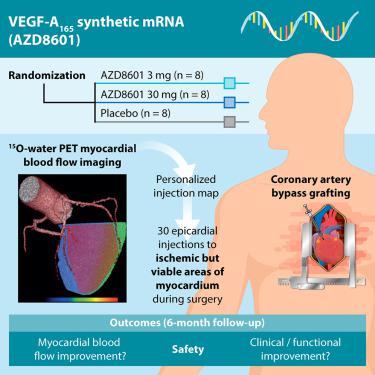
Therapeutic angiogenesis may improve outcomes in patients with coronary artery disease undergoing surgical revascularization. Angiogenic factors may promote blood vessel growth and regenerate regions of ischemic but viable myocardium. Previous clinical trials of vascular endothelial growth factor A (VEGF-A) gene therapy with DNA or viral vectors demonstrated safety but not efficacy. AZD8601 is VEGF-A165 mRNA formulated in biocompatible citrate-buffered saline and optimized for high-efficiency VEGF-A expression with minimal innate immune response. EPICCURE is an ongoing randomized, double-blind, placebo-controlled study of the safety of AZD8601 in patients with moderately decreased left ventricular function (ejection fraction 30%–50%) undergoing elective coronary artery bypass surgery. AZD8601 3 mg, 30 mg, or placebo is administered as 30 epicardial injections in a 10-min extension of cardioplegia. Injections are targeted to ischemic but viable myocardial regions in each patient using quantitative 15O-water positron emission tomography (PET) imaging (stress myocardial blood flow < 2.3 mL/g/min; resting myocardial blood flow > 0.6 mL/g/min). Improvement in regional and global myocardial blood flow quantified with 15O-water PET is an exploratory efficacy outcome, together with echocardiographic, clinical, functional, and biomarker measures. EPICCURE combines high-efficiency delivery with quantitative targeting and follow-up for robust assessment of the safety and exploratory efficacy of VEGF-A mRNA angiogenesis (ClinicalTrials.gov: NCT03370887).
中文翻译:

冠状动脉搭桥术患者中编码VEGF-A的合成mRNA:2a期临床试验的设计。
治疗性血管生成可改善接受外科血管重建术的冠心病患者的预后。血管生成因子可促进血管生长并再生缺血但可存活的心肌区域。以前使用DNA或病毒载体进行的血管内皮生长因子A(VEGF-A)基因治疗的临床试验证明了安全性,但没有疗效。AZD8601是VEGF-A 165在生物相容性柠檬酸盐缓冲盐溶液中配制的mRNA,并针对高效VEGF-A表达进行了优化,而固有免疫反应极少。EPICCURE是一项正在进行的随机,双盲,安慰剂对照研究,用于接受择期冠状动脉搭桥手术的左心室功能中度下降(射血分数为30%–50%)的患者AZD8601的安全性。AZD8601 3毫克,30毫克或安慰剂以30次心外膜注射剂的形式在延长10分钟的心脏停搏中给药。使用定量的15剂,将注射针对每个患者的缺血但可行的心肌区域O水正电子发射断层扫描(PET)成像(压力心肌血流量<2.3 mL / g / min;静息心肌血流量> 0.6 mL / g / min)。用15 O-水PET定量测定的局部和整体心肌血流量的改善是一项探索性疗效结果,同时还结合了超声心动图,临床,功能和生物标志物措施。EPICCURE将高效递送与定量靶向和随访相结合,可对VEGF-A mRNA血管生成的安全性和探索性功效进行有力的评估(ClinicalTrials.gov:NCT03370887)。









































 京公网安备 11010802027423号
京公网安备 11010802027423号