当前位置:
X-MOL 学术
›
J. Mol. Struct.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Formamidine-based thiuram disulfides: Synthesis, structural characterization, biological studies, and preliminary cheminformatics evaluation
Journal of Molecular Structure ( IF 4.0 ) Pub Date : 2020-11-01 , DOI: 10.1016/j.molstruc.2020.128553 Segun D. Oladipo , Fisayo A. Olotu , Mahmoud Soliman , Chunderika Mocktar , Bernard Omondi
Journal of Molecular Structure ( IF 4.0 ) Pub Date : 2020-11-01 , DOI: 10.1016/j.molstruc.2020.128553 Segun D. Oladipo , Fisayo A. Olotu , Mahmoud Soliman , Chunderika Mocktar , Bernard Omondi
|
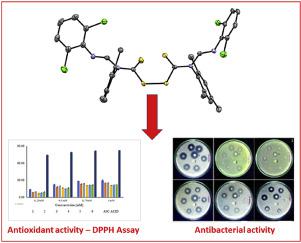
Abstract In this study, we present the synthesis and biological evaluation of a series of formamidine-based thiuram disulfides using computational tools. Six compounds were synthesized via, the oxidation of formamidine-based dithiocarbamates. These were, N,N′-bis(2,6-dimethylphenyl)formamidine dithiocarbamate (L1), N,N′-bis(2,6-disopropylphenyl) formamidine dithiocarbamate (L2), N,N′-dimesitylformamidine dithiocarbamate (L3), N’-(2,6-dichlorophenyl-N-(2,6-dimethylphenyl)formamidine dithiocarbamate (L4), N’-(2,6-dichlorophenyl)-N-(2,6-diisopropylphenyl)formamidine dithiocarbamate (L5) and N’-(2,6-dichlorophenyl)-N-mesitylformamidine dithiocarbamate (L6). The oxidation of the dithiocarbamates with iodine produced sulfur-sulfur coupling compounds L1’ (1), L2’ (2), L3’ (3), L4’ (4), L5’ (5) and L6’ (6). All the compounds were characterized using UV–vis, FT-IR, 1H and 13C NMR, mass spectrometry and by elemental analysis. In addition, the single crystal structures of compounds 1, 2, 4 and 5 are reported and confirm the coupling of formamidine dithiocarbamates moieties in 1–6. In vitro antimicrobial study revealed that all the compounds displayed moderate to good activities against Gram-negative bacteria, Escherichia coli, Salmonella typhimurium, Klebsiella pneumoniae, and Pseudomonas aeruginosa, but none of the six were active against Gram-positive bacteria, methicillin-resistant Staphylococcus aureus (MRSA) and Staphylococcus aureus. Compounds 4 and 5 were found to be more active than ciprofloxacin against S. typhimurium and all compounds exhibited poor antioxidant activity when compared to ascorbic acid. Pharmacological estimations of 1–6 revealed that all the compounds had minimal violations of Lipinski’s rule but exhibited inclinations to be orally bioavailable and less toxic.
中文翻译:

基于甲脒的秋兰姆二硫化物:合成、结构表征、生物学研究和初步化学信息学评估
摘要 在这项研究中,我们使用计算工具介绍了一系列基于甲脒的二硫化秋兰姆的合成和生物学评价。通过甲脒基二硫代氨基甲酸酯的氧化合成了六种化合物。它们是,N,N'-双(2,6-二甲基苯基)甲脒二硫代氨基甲酸酯(L1)、N,N'-双(2,6-二异丙基苯基)甲脒二硫代氨基甲酸酯(L2)、N,N'-二甲基甲脒二硫代氨基甲酸酯(L3 ), N'-(2,6-二氯苯基-N-(2,6-二甲基苯基)甲脒二硫代氨基甲酸酯 (L4), N'-(2,6-二氯苯基)-N-(2,6-二异丙基苯基)甲脒二硫代氨基甲酸酯 ( L5) 和 N'-(2,6-二氯苯基)-N-甲基甲脒二硫代氨基甲酸酯 (L6)。二硫代氨基甲酸酯与碘的氧化产生硫-硫偶联化合物 L1' (1), L2' (2), L3' ( 3)、L4' (4)、L5' (5) 和 L6' (6)。所有化合物均使用 UV-vis、FT-IR、1H 和 13C NMR、质谱和元素分析进行表征。此外,报告了化合物 1、2、4 和 5 的单晶结构,并证实了甲脒二硫代氨基甲酸酯部分在 1-6 中的偶联。体外抗菌研究表明,所有化合物对革兰氏阴性菌、大肠杆菌、鼠伤寒沙门氏菌、肺炎克雷伯菌和铜绿假单胞菌均表现出中等至良好的活性,但六种化合物对革兰氏阳性菌、耐甲氧西林葡萄球菌均无活性金黄色葡萄球菌 (MRSA) 和金黄色葡萄球菌。发现化合物 4 和 5 对鼠伤寒沙门氏菌的活性高于环丙沙星,并且与抗坏血酸相比,所有化合物的抗氧化活性都很差。
更新日期:2020-11-01
中文翻译:

基于甲脒的秋兰姆二硫化物:合成、结构表征、生物学研究和初步化学信息学评估
摘要 在这项研究中,我们使用计算工具介绍了一系列基于甲脒的二硫化秋兰姆的合成和生物学评价。通过甲脒基二硫代氨基甲酸酯的氧化合成了六种化合物。它们是,N,N'-双(2,6-二甲基苯基)甲脒二硫代氨基甲酸酯(L1)、N,N'-双(2,6-二异丙基苯基)甲脒二硫代氨基甲酸酯(L2)、N,N'-二甲基甲脒二硫代氨基甲酸酯(L3 ), N'-(2,6-二氯苯基-N-(2,6-二甲基苯基)甲脒二硫代氨基甲酸酯 (L4), N'-(2,6-二氯苯基)-N-(2,6-二异丙基苯基)甲脒二硫代氨基甲酸酯 ( L5) 和 N'-(2,6-二氯苯基)-N-甲基甲脒二硫代氨基甲酸酯 (L6)。二硫代氨基甲酸酯与碘的氧化产生硫-硫偶联化合物 L1' (1), L2' (2), L3' ( 3)、L4' (4)、L5' (5) 和 L6' (6)。所有化合物均使用 UV-vis、FT-IR、1H 和 13C NMR、质谱和元素分析进行表征。此外,报告了化合物 1、2、4 和 5 的单晶结构,并证实了甲脒二硫代氨基甲酸酯部分在 1-6 中的偶联。体外抗菌研究表明,所有化合物对革兰氏阴性菌、大肠杆菌、鼠伤寒沙门氏菌、肺炎克雷伯菌和铜绿假单胞菌均表现出中等至良好的活性,但六种化合物对革兰氏阳性菌、耐甲氧西林葡萄球菌均无活性金黄色葡萄球菌 (MRSA) 和金黄色葡萄球菌。发现化合物 4 和 5 对鼠伤寒沙门氏菌的活性高于环丙沙星,并且与抗坏血酸相比,所有化合物的抗氧化活性都很差。











































 京公网安备 11010802027423号
京公网安备 11010802027423号