Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics ( IF 2.5 ) Pub Date : 2020-06-02 , DOI: 10.1016/j.bbapap.2020.140464 Rina Okuwaki 1 , Iori Shinmura 1 , Shiki Morita 1 , Akimasa Matsugami 2 , Fumiaki Hayashi 2 , Yuji Goto 3 , Chiaki Nishimura 4
|
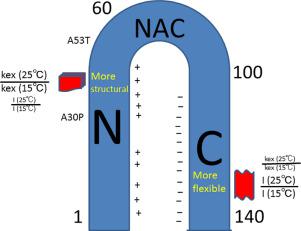
The residual solution structures of two alpha-synuclein mutants, A30P and A53T, observed in family members of patients with Parkinson's disease were compared with that of wild-type by NMR. The A53T substitution had been shown to accelerate fibril formation of alpha-synuclein, whereas the A30P mutation has the negative and positive effects on the formation of the fibril and spherical oligomer, respectively. The remaining structure was analyzed via amide-proton exchange and signal intensity measurements using NMR. Amide-proton exchange was used for both the calculation of kex values and ratio of kex at different temperatures. Effects of the A30P (N-terminal region) mutation were observed at the C-terminal region as a more flexible structure, suggesting that long-range interactions exist between the N- and C-terminal regions in alpha-synuclein. In addition, the N-terminal region adopted a more rigid structure in the A53T and A30P mutants than in the wild-type. It was concluded that the structural change caused by the mutations is related to the formation of a beta-hairpin at the initiation site of the N-terminal core structure. Furthermore, the signal intensity was used to estimate the rigidity of the structure. Higher signal intensities were observed for A30P at the 112, 113, and 116 C-terminal residues, suggesting that this region adopts more flexible structure. The ratio of the intensities at different temperatures indicated more flexible or rigid structures in the N-terminal region of A30P than in that of wild-type. Thus, using different approaches and temperatures is a good method to analyze residual structure in intrinsically disordered proteins.
中文翻译:

通过酰胺-质子交换和NMR信号强度分析了α-突触核蛋白的不同残基和无序结构。
通过NMR比较了在帕金森氏病患者的家庭成员中观察到的两个α-突触核蛋白突变体A30P和A53T的残留溶液结构与野生型的残留溶液结构。已显示A53T取代可加速α-突触核蛋白的原纤维形成,而A30P突变分别对原纤维和球形低聚物的形成具有负面和正面影响。通过酰胺-质子交换和使用NMR的信号强度测量来分析剩余的结构。酰胺-质子交换用于计算k ex值和k ex的比率在不同的温度下。在C末端区域观察到了A30P(N末端区域)突变的影响,这是一种更灵活的结构,这表明在α-突触核蛋白的N末端和C末端区域之间存在着长距离相互作用。此外,与野生型相比,A53T和A30P突变体的N端区域具有更刚性的结构。结论是,由突变引起的结构变化与在N末端核心结构的起始位点形成β-发夹有关。此外,信号强度用于估算结构的刚度。在112、113和116 C端残基处观察到更高的信号强度,表明该区域采用了更灵活的结构。在不同温度下的强度比表明,A30P的N端区域比野生型具有更多的柔性或刚性结构。因此,使用不同的方法和温度是分析内在无序蛋白中残留结构的好方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号