当前位置:
X-MOL 学术
›
CrystEngComm
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electron density based analysis of N–H⋯OC hydrogen bonds and electrostatic interaction energies in high-resolution secondary protein structures: insights from quantum crystallographic approaches
CrystEngComm ( IF 2.6 ) Pub Date : 2020-06-01 , DOI: 10.1039/d0ce00577k Suman K. Mandal 1, 2, 3, 4, 5 , Benoît Guillot 6, 7, 8, 9, 10 , Parthapratim Munshi 1, 2, 3, 4, 5
CrystEngComm ( IF 2.6 ) Pub Date : 2020-06-01 , DOI: 10.1039/d0ce00577k Suman K. Mandal 1, 2, 3, 4, 5 , Benoît Guillot 6, 7, 8, 9, 10 , Parthapratim Munshi 1, 2, 3, 4, 5
Affiliation
|
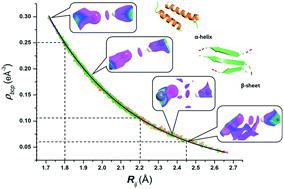
In proteins, the main-chain N–H⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C hydrogen bonds (HBs) play a crucial role in the formation of α-helices and β-sheets. Accurate analysis of such hydrogen bonds and their electrostatic interaction energies is essential for studying binding interactions and for better understanding of the energetics involved in protein folding. Here, we studied 22 high-resolution (0.87 Å to 0.48 Å) secondary protein structures (4.7 kDa to 54.5 kDa) from the RCSB PDB and performed topological analyses of 1443 N–H⋯O
C hydrogen bonds (HBs) play a crucial role in the formation of α-helices and β-sheets. Accurate analysis of such hydrogen bonds and their electrostatic interaction energies is essential for studying binding interactions and for better understanding of the energetics involved in protein folding. Here, we studied 22 high-resolution (0.87 Å to 0.48 Å) secondary protein structures (4.7 kDa to 54.5 kDa) from the RCSB PDB and performed topological analyses of 1443 N–H⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C HBs (750 in α-helices and 693 in β-sheets) using the multipole analysis based experimental electron densities as transferred from the ELMAM2 database. This is the first study of its kind involving by far the largest number of high-resolution protein structures and HBs from both α-helices and β-sheets. Further, based on the accurate estimation of the electrostatic interaction energies, the excellent correlations with various topological parameters have been demonstrated. The excellent correlations have also been observed between the topological parameters. Thereby, we identified the limiting values of the topological parameters and the electrostatic interaction energies to establish the presence of the true N–H⋯O
C HBs (750 in α-helices and 693 in β-sheets) using the multipole analysis based experimental electron densities as transferred from the ELMAM2 database. This is the first study of its kind involving by far the largest number of high-resolution protein structures and HBs from both α-helices and β-sheets. Further, based on the accurate estimation of the electrostatic interaction energies, the excellent correlations with various topological parameters have been demonstrated. The excellent correlations have also been observed between the topological parameters. Thereby, we identified the limiting values of the topological parameters and the electrostatic interaction energies to establish the presence of the true N–H⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C HBs in protein main-chains via quantitative and qualitative analyses of electron densities using quantum crystallographic approaches – the quantum theory of atoms in molecules (QTAIM) and the noncovalent interaction (NCI) index.
C HBs in protein main-chains via quantitative and qualitative analyses of electron densities using quantum crystallographic approaches – the quantum theory of atoms in molecules (QTAIM) and the noncovalent interaction (NCI) index.
中文翻译:

基于电子密度的高分辨率二级蛋白质结构中N–H⋯OC氢键和静电相互作用能的分析:量子晶体学方法的见解
在蛋白质中,主链N–H⋯O![[双键,长度为m-破折号]](https://www.rsc.org/images/entities/char_e001.gif) C氢键(HBs)在α-螺旋和β-折叠层的形成中起关键作用。对此类氢键及其静电相互作用能的准确分析对于研究结合相互作用以及更好地理解蛋白质折叠所涉及的能量学至关重要。在这里,我们研究了来自RCSB PDB的22种高分辨率(0.87Å至0.48Å)二级蛋白质结构(4.7 kDa至54.5 kDa),并对1443 N–H⋯O
C氢键(HBs)在α-螺旋和β-折叠层的形成中起关键作用。对此类氢键及其静电相互作用能的准确分析对于研究结合相互作用以及更好地理解蛋白质折叠所涉及的能量学至关重要。在这里,我们研究了来自RCSB PDB的22种高分辨率(0.87Å至0.48Å)二级蛋白质结构(4.7 kDa至54.5 kDa),并对1443 N–H⋯O ![[双键,长度为m-破折号]](https://www.rsc.org/images/entities/char_e001.gif) C HBs(750个α螺旋和693个)进行了拓扑分析在β-折叠中),使用基于多极分析的实验电子密度(从ELMAM2转移过来)数据库。这是迄今为止此类研究中的首次,涉及来自α-螺旋和β-折叠的高分辨率蛋白质结构和HBs最多。此外,基于对静电相互作用能的精确估计,已经证明了与各种拓扑参数的极好的相关性。拓扑参数之间也观察到了极好的相关性。由此,我们确定了拓扑参数和静电相互作用能的限制值以建立真正N-H⋯O3存在
C HBs(750个α螺旋和693个)进行了拓扑分析在β-折叠中),使用基于多极分析的实验电子密度(从ELMAM2转移过来)数据库。这是迄今为止此类研究中的首次,涉及来自α-螺旋和β-折叠的高分辨率蛋白质结构和HBs最多。此外,基于对静电相互作用能的精确估计,已经证明了与各种拓扑参数的极好的相关性。拓扑参数之间也观察到了极好的相关性。由此,我们确定了拓扑参数和静电相互作用能的限制值以建立真正N-H⋯O3存在![[双键,长度为m-破折号]](https://www.rsc.org/images/entities/char_e001.gif) ÇHBs抗体在蛋白质主链经由 使用量子晶体学方法对电子密度进行定量和定性分析–分子中原子的量子理论(QTAIM)和非共价相互作用(NCI)指数。
ÇHBs抗体在蛋白质主链经由 使用量子晶体学方法对电子密度进行定量和定性分析–分子中原子的量子理论(QTAIM)和非共价相互作用(NCI)指数。
更新日期:2020-07-06
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C hydrogen bonds (HBs) play a crucial role in the formation of α-helices and β-sheets. Accurate analysis of such hydrogen bonds and their electrostatic interaction energies is essential for studying binding interactions and for better understanding of the energetics involved in protein folding. Here, we studied 22 high-resolution (0.87 Å to 0.48 Å) secondary protein structures (4.7 kDa to 54.5 kDa) from the RCSB PDB and performed topological analyses of 1443 N–H⋯O
C hydrogen bonds (HBs) play a crucial role in the formation of α-helices and β-sheets. Accurate analysis of such hydrogen bonds and their electrostatic interaction energies is essential for studying binding interactions and for better understanding of the energetics involved in protein folding. Here, we studied 22 high-resolution (0.87 Å to 0.48 Å) secondary protein structures (4.7 kDa to 54.5 kDa) from the RCSB PDB and performed topological analyses of 1443 N–H⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C HBs (750 in α-helices and 693 in β-sheets) using the multipole analysis based experimental electron densities as transferred from the ELMAM2 database. This is the first study of its kind involving by far the largest number of high-resolution protein structures and HBs from both α-helices and β-sheets. Further, based on the accurate estimation of the electrostatic interaction energies, the excellent correlations with various topological parameters have been demonstrated. The excellent correlations have also been observed between the topological parameters. Thereby, we identified the limiting values of the topological parameters and the electrostatic interaction energies to establish the presence of the true N–H⋯O
C HBs (750 in α-helices and 693 in β-sheets) using the multipole analysis based experimental electron densities as transferred from the ELMAM2 database. This is the first study of its kind involving by far the largest number of high-resolution protein structures and HBs from both α-helices and β-sheets. Further, based on the accurate estimation of the electrostatic interaction energies, the excellent correlations with various topological parameters have been demonstrated. The excellent correlations have also been observed between the topological parameters. Thereby, we identified the limiting values of the topological parameters and the electrostatic interaction energies to establish the presence of the true N–H⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C HBs in protein main-chains via quantitative and qualitative analyses of electron densities using quantum crystallographic approaches – the quantum theory of atoms in molecules (QTAIM) and the noncovalent interaction (NCI) index.
C HBs in protein main-chains via quantitative and qualitative analyses of electron densities using quantum crystallographic approaches – the quantum theory of atoms in molecules (QTAIM) and the noncovalent interaction (NCI) index.
中文翻译:

基于电子密度的高分辨率二级蛋白质结构中N–H⋯OC氢键和静电相互作用能的分析:量子晶体学方法的见解
在蛋白质中,主链N–H⋯O
![[双键,长度为m-破折号]](https://www.rsc.org/images/entities/char_e001.gif) C氢键(HBs)在α-螺旋和β-折叠层的形成中起关键作用。对此类氢键及其静电相互作用能的准确分析对于研究结合相互作用以及更好地理解蛋白质折叠所涉及的能量学至关重要。在这里,我们研究了来自RCSB PDB的22种高分辨率(0.87Å至0.48Å)二级蛋白质结构(4.7 kDa至54.5 kDa),并对1443 N–H⋯O
C氢键(HBs)在α-螺旋和β-折叠层的形成中起关键作用。对此类氢键及其静电相互作用能的准确分析对于研究结合相互作用以及更好地理解蛋白质折叠所涉及的能量学至关重要。在这里,我们研究了来自RCSB PDB的22种高分辨率(0.87Å至0.48Å)二级蛋白质结构(4.7 kDa至54.5 kDa),并对1443 N–H⋯O ![[双键,长度为m-破折号]](https://www.rsc.org/images/entities/char_e001.gif) C HBs(750个α螺旋和693个)进行了拓扑分析在β-折叠中),使用基于多极分析的实验电子密度(从ELMAM2转移过来)数据库。这是迄今为止此类研究中的首次,涉及来自α-螺旋和β-折叠的高分辨率蛋白质结构和HBs最多。此外,基于对静电相互作用能的精确估计,已经证明了与各种拓扑参数的极好的相关性。拓扑参数之间也观察到了极好的相关性。由此,我们确定了拓扑参数和静电相互作用能的限制值以建立真正N-H⋯O3存在
C HBs(750个α螺旋和693个)进行了拓扑分析在β-折叠中),使用基于多极分析的实验电子密度(从ELMAM2转移过来)数据库。这是迄今为止此类研究中的首次,涉及来自α-螺旋和β-折叠的高分辨率蛋白质结构和HBs最多。此外,基于对静电相互作用能的精确估计,已经证明了与各种拓扑参数的极好的相关性。拓扑参数之间也观察到了极好的相关性。由此,我们确定了拓扑参数和静电相互作用能的限制值以建立真正N-H⋯O3存在![[双键,长度为m-破折号]](https://www.rsc.org/images/entities/char_e001.gif) ÇHBs抗体在蛋白质主链经由 使用量子晶体学方法对电子密度进行定量和定性分析–分子中原子的量子理论(QTAIM)和非共价相互作用(NCI)指数。
ÇHBs抗体在蛋白质主链经由 使用量子晶体学方法对电子密度进行定量和定性分析–分子中原子的量子理论(QTAIM)和非共价相互作用(NCI)指数。











































 京公网安备 11010802027423号
京公网安备 11010802027423号