当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Access to Spirocyclic Benzothiophenones with Multiple Stereocenters via an Organocatalytic Cascade Reaction.
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-06-01 , DOI: 10.1021/acs.joc.0c00882 Bedřich Formánek 1 , Jiří Tauchman 1 , Ivana Císařová 2 , Jan Veselý 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-06-01 , DOI: 10.1021/acs.joc.0c00882 Bedřich Formánek 1 , Jiří Tauchman 1 , Ivana Císařová 2 , Jan Veselý 1
Affiliation
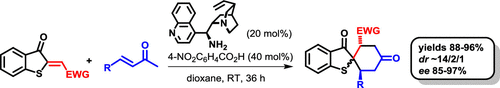
|
The present report describes an organocatalytic cascade reaction between 2-alkylidene benzo[b]thiophenone derivatives and enones in the presence of the Cinchona alkaloid amine. Spirobenzothiophenonic cyclohexane derivatives containing three stereocenters were prepared via one-step synthesis in yields ranging from 88 to 96% and in enantioselectivities (enantiomeric excess (ee)) ranging from 85 to 97%, with diastereoselectivities of approximately 14/2/1. Therefore, this method provides an efficient route for the synthesis of a new class of optically active 2-spirobenzothiophenones.
中文翻译:

通过有机催化级联反应获得具有多个立体中心的螺环苯并噻吩酮。
本报告描述了在金鸡纳生物碱胺存在下2-亚烷基苯并[ b ]噻吩酮衍生物与烯酮之间的有机催化级联反应。通过一步合成,制备了包含三个立体中心的螺苯并噻吩苯甲酸环己烷衍生物,其产率为88%至96%,对映选择性(对映体过量(ee))为85至97%,非对映选择性约为14/2/1。因此,该方法为合成新型的光学活性的2-螺苯并噻吩酮提供了有效的途径。
更新日期:2020-07-02
中文翻译:

通过有机催化级联反应获得具有多个立体中心的螺环苯并噻吩酮。
本报告描述了在金鸡纳生物碱胺存在下2-亚烷基苯并[ b ]噻吩酮衍生物与烯酮之间的有机催化级联反应。通过一步合成,制备了包含三个立体中心的螺苯并噻吩苯甲酸环己烷衍生物,其产率为88%至96%,对映选择性(对映体过量(ee))为85至97%,非对映选择性约为14/2/1。因此,该方法为合成新型的光学活性的2-螺苯并噻吩酮提供了有效的途径。











































 京公网安备 11010802027423号
京公网安备 11010802027423号