当前位置:
X-MOL 学术
›
Macromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Functionalizing Polystyrene with N-Alicyclic Piperidine-Based Cations via Friedel-Crafts Alkylation for Highly Alkali-Stable Anion-Exchange Membranes.
Macromolecules ( IF 5.5 ) Pub Date : 2020-06-01 , DOI: 10.1021/acs.macromol.0c00201 Joel S Olsson 1 , Thanh Huong Pham 1 , Patric Jannasch 1
Macromolecules ( IF 5.5 ) Pub Date : 2020-06-01 , DOI: 10.1021/acs.macromol.0c00201 Joel S Olsson 1 , Thanh Huong Pham 1 , Patric Jannasch 1
Affiliation
|
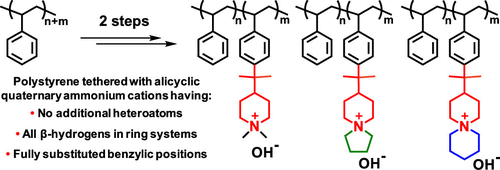
Different anion-exchange membranes (AEMs) based on polystyrene (PS)-carrying benzyltrimethyl ammonium cations are currently being developed for use in alkaline fuel cells and water electrolyzers. However, the stability in relation to these state-of-the-art cations needs to be further improved. Here, we introduce highly alkali-stable mono- and spirocyclic piperidine-based cations onto PS by first performing a superacid-mediated Friedel–Crafts alkylation using 2-(piperidine-4-yl)propane-2-ol. This is followed by quaternization of the piperidine rings either using iodomethane to produce N,N-dimethyl piperidinium cations or by cyclo-quaternizations using 1,5-dibromopentane and 1,4-dibromobutane, respectively, to obtain N-spirocyclic quaternary ammonium cations. Thus, it is possible to functionalize up to 27% of the styrene units with piperidine rings and subsequently achieve complete quaternization. The synthetic approach ensures that all of the sensitive β-hydrogens of the cations are present in ring structures to provide high stability. AEMs based on these polymers show high alkaline stability and less than 5% ionic loss was observed by 1H NMR spectroscopy after 30 days in 2 M aq NaOH at 90 °C. AEMs functionalized with N,N-dimethyl piperidinium cations show higher stability than the ones carrying N-spirocyclic quaternary ammonium. Careful analysis of the latter revealed that the rings formed in the cyclo-quaternization are more prone to degrade via Hofmann elimination than the rings introduced in the Friedel–Crafts reaction. AEMs with an ion-exchange capacity of 1.5 mequiv g–1 reach a hydroxide conductivity of 106 mS cm–1 at 80 °C under fully hydrated conditions. The AEMs are further tuned and improved by blending with polybenzimidazole (PBI). For example, an AEM containing 2 wt % PBI shows reduced water uptake and much improved robustness during handling and reaches 71 mS cm–1 at 80 °C. The study demonstrates that the critical alkaline stability of PS-containing AEMs can be significantly enhanced by replacing the benchmark benzyltrimethyl ammonium cations with N-alicyclic piperidine-based cations.
中文翻译:

通过N脂环族哌啶基阳离子通过Friedel-Crafts烷基化对高度碱稳定的阴离子交换膜进行功能化的聚苯乙烯。
目前正在开发基于携带聚苯乙烯(PS)的苄基三甲基铵阳离子的不同阴离子交换膜(AEM),用于碱性燃料电池和水电解槽。但是,与这些最新阳离子相关的稳定性需要进一步提高。在这里,我们首先通过使用2-(哌啶-4-基)丙烷-2-醇进行超强酸介导的Friedel-Crafts烷基化,将高度碱稳定的单环和螺环哌啶基阳离子引入PS。然后使用碘代甲烷生成N,N-二甲基哌啶鎓阳离子对哌啶环进行季铵化,或分别使用1,5-二溴戊烷和1,4-二溴丁烷进行环季铵化,以获得N-螺环季铵阳离子。因此,有可能用哌啶环官能化多达27%的苯乙烯单元,随后实现完全季铵化。合成方法可确保阳离子的所有敏感β-氢均存在于环结构中,以提供高稳定性。基于这些聚合物的AEM在90°C下在2 M NaOH水溶液中放置30天后,通过1 H NMR光谱显示出较高的碱性稳定性和小于5%的离子损失。用N,N-二甲基哌啶鎓阳离子官能化的AEM表现出比带有N-螺环季铵盐的AEM高的稳定性。对后者的仔细分析表明,在环季铵化反应中形成的环更容易降解通过霍夫曼消除,比在Friedel-Crafts反应中引入的环要强。在完全水合条件下,离子交换容量为1.5 mequiv g –1的AEM在80°C时达到106 mS cm –1的氢氧化物电导率。通过与聚苯并咪唑(PBI)共混,可以进一步调整和改进AEM。例如,含有2 wt%PBI的AEM在处理过程中显示出减少的吸水率和坚固性,并在80°C时达到71 mS cm –1。该研究表明,通过将N-脂环族哌啶基阳离子替代基准苄基三甲基铵阳离子,可以显着提高含PS的AEM的关键碱性稳定性。
更新日期:2020-06-23
中文翻译:

通过N脂环族哌啶基阳离子通过Friedel-Crafts烷基化对高度碱稳定的阴离子交换膜进行功能化的聚苯乙烯。
目前正在开发基于携带聚苯乙烯(PS)的苄基三甲基铵阳离子的不同阴离子交换膜(AEM),用于碱性燃料电池和水电解槽。但是,与这些最新阳离子相关的稳定性需要进一步提高。在这里,我们首先通过使用2-(哌啶-4-基)丙烷-2-醇进行超强酸介导的Friedel-Crafts烷基化,将高度碱稳定的单环和螺环哌啶基阳离子引入PS。然后使用碘代甲烷生成N,N-二甲基哌啶鎓阳离子对哌啶环进行季铵化,或分别使用1,5-二溴戊烷和1,4-二溴丁烷进行环季铵化,以获得N-螺环季铵阳离子。因此,有可能用哌啶环官能化多达27%的苯乙烯单元,随后实现完全季铵化。合成方法可确保阳离子的所有敏感β-氢均存在于环结构中,以提供高稳定性。基于这些聚合物的AEM在90°C下在2 M NaOH水溶液中放置30天后,通过1 H NMR光谱显示出较高的碱性稳定性和小于5%的离子损失。用N,N-二甲基哌啶鎓阳离子官能化的AEM表现出比带有N-螺环季铵盐的AEM高的稳定性。对后者的仔细分析表明,在环季铵化反应中形成的环更容易降解通过霍夫曼消除,比在Friedel-Crafts反应中引入的环要强。在完全水合条件下,离子交换容量为1.5 mequiv g –1的AEM在80°C时达到106 mS cm –1的氢氧化物电导率。通过与聚苯并咪唑(PBI)共混,可以进一步调整和改进AEM。例如,含有2 wt%PBI的AEM在处理过程中显示出减少的吸水率和坚固性,并在80°C时达到71 mS cm –1。该研究表明,通过将N-脂环族哌啶基阳离子替代基准苄基三甲基铵阳离子,可以显着提高含PS的AEM的关键碱性稳定性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号