当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure-Function Studies of the Antibiotic Target l,l-Diaminopimelate Aminotransferase from Verrucomicrobium spinosum Reveal an Unusual Oligomeric Structure.
Biochemistry ( IF 2.9 ) Pub Date : 2020-06-01 , DOI: 10.1021/acs.biochem.0c00185 Anthony W Weatherhead 1 , Jennifer M Crowther 1 , Christopher R Horne 1 , Yanxiang Meng 1 , David Coombes 1 , Michael J Currie 1 , Serena A J Watkin 1 , Lily E Adams 2 , Anutthaman Parthasarathy 2 , Renwick C J Dobson 1, 3 , André O Hudson 2
Biochemistry ( IF 2.9 ) Pub Date : 2020-06-01 , DOI: 10.1021/acs.biochem.0c00185 Anthony W Weatherhead 1 , Jennifer M Crowther 1 , Christopher R Horne 1 , Yanxiang Meng 1 , David Coombes 1 , Michael J Currie 1 , Serena A J Watkin 1 , Lily E Adams 2 , Anutthaman Parthasarathy 2 , Renwick C J Dobson 1, 3 , André O Hudson 2
Affiliation
|
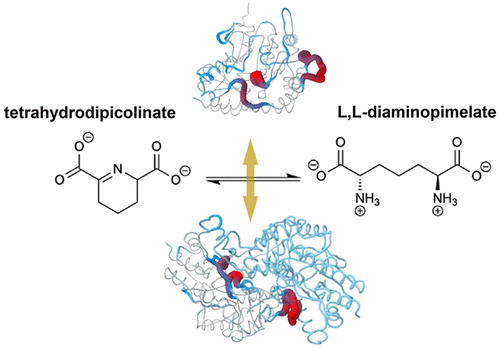
While humans lack the biosynthetic pathways for meso-diaminopimelate and l-lysine, they are essential for bacterial survival and are therefore attractive targets for antibiotics. It was recently discovered that members of the Chlamydia family utilize a rare aminotransferase route of the l-lysine biosynthetic pathway, thus offering a new enzymatic drug target. Here we characterize diaminopimelate aminotransferase from Verrucomicrobium spinosum (VsDapL), a nonpathogenic model bacterium for Chlamydia trachomatis. Complementation experiments verify that the V. spinosum dapL gene encodes a bona fide diaminopimelate aminotransferase, because the gene rescues an Escherichia coli strain that is auxotrophic for meso-diaminopimelate. Kinetic studies show that VsDapL follows a Michaelis–Menten mechanism, with a KMapp of 4.0 mM toward its substrate l,l-diaminopimelate. The kcat (0.46 s–1) and the kcat/KM (115 s–1 M–1) are somewhat lower than values for other diaminopimelate aminotransferases. Moreover, whereas other studied DapL orthologs are dimeric, sedimentation velocity experiments demonstrate that VsDapL exists in a monomer–dimer self-association, with a KD2-1 of 7.4 μM. The 2.25 Å resolution crystal structure presents the canonical dimer of chalice-shaped monomers, and small-angle X-ray scattering experiments confirm the dimer in solution. Sequence and structural alignments reveal that active site residues important for activity are conserved in VsDapL, despite the lower activity compared to those of other DapL homologues. Although the dimer interface buries 18% of the total surface area, several loops that contribute to the interface and active site, notably the L1, L2, and L5 loops, are highly mobile, perhaps explaining the unstable dimer and lower catalytic activity. Our kinetic, biophysical, and structural characterization can be used to inform the development of antibiotics.
中文翻译:

源自疣状疣疣的抗生素靶标I,1-二氨基庚二酸酯氨基转移酶的结构功能研究揭示了异常的寡聚结构。
尽管人类缺乏中消旋-二氨基庚二酸酯和1-赖氨酸的生物合成途径,但它们对于细菌存活至关重要,因此是抗生素的诱人靶标。最近发现衣原体家族的成员利用1-赖氨酸生物合成途径的罕见的氨基转移酶途径,从而提供了新的酶促药物靶标。在这里,我们描述了来自沙眼疣疣(Vs DapL)的沙眼衣原体的非致病性模型细菌的二氨基庚二酸酯转氨酶。补充实验验证了棘孢菌dapL该基因编码一个真正的二氨基庚二酸酯氨基转移酶,因为该基因拯救了对内消旋的二氨基庚二酸酯营养缺陷的大肠杆菌菌株。动力学研究显示,Vs的DAPL遵循米氏机构,具有ķ中号应用4.0mM的朝向它的底物升,升-diaminopimelate。所述ķ猫(0.46小号-1)和ķ猫/ ķ中号(115号-1中号-1)略低于其他二氨基庚二酸酯氨基转移酶的值。此外,尽管其他研究的DapL直系同源物是二聚体,但沉降速度实验表明Vs DapL存在于单体-二聚体自缔合中,K D 2-1为7.4μM。2.25Å分辨率的晶体结构呈现出杯状单体的典型二聚体,小角X射线散射实验证实了溶液中的二聚体。序列和结构比对显示,对活性重要的活性位点残基在Vs中是保守的尽管DapL的活性比其他DapL同源物的活性低。尽管二聚体界面占据了总表面积的18%,但对界面和活性位点有贡献的多个环(尤其是L1,L2和L5环)具有很高的移动性,这也许可以解释不稳定的二聚体和较低的催化活性。我们的动力学,生物物理和结构表征可用于告知抗生素的开发。
更新日期:2020-06-23
中文翻译:

源自疣状疣疣的抗生素靶标I,1-二氨基庚二酸酯氨基转移酶的结构功能研究揭示了异常的寡聚结构。
尽管人类缺乏中消旋-二氨基庚二酸酯和1-赖氨酸的生物合成途径,但它们对于细菌存活至关重要,因此是抗生素的诱人靶标。最近发现衣原体家族的成员利用1-赖氨酸生物合成途径的罕见的氨基转移酶途径,从而提供了新的酶促药物靶标。在这里,我们描述了来自沙眼疣疣(Vs DapL)的沙眼衣原体的非致病性模型细菌的二氨基庚二酸酯转氨酶。补充实验验证了棘孢菌dapL该基因编码一个真正的二氨基庚二酸酯氨基转移酶,因为该基因拯救了对内消旋的二氨基庚二酸酯营养缺陷的大肠杆菌菌株。动力学研究显示,Vs的DAPL遵循米氏机构,具有ķ中号应用4.0mM的朝向它的底物升,升-diaminopimelate。所述ķ猫(0.46小号-1)和ķ猫/ ķ中号(115号-1中号-1)略低于其他二氨基庚二酸酯氨基转移酶的值。此外,尽管其他研究的DapL直系同源物是二聚体,但沉降速度实验表明Vs DapL存在于单体-二聚体自缔合中,K D 2-1为7.4μM。2.25Å分辨率的晶体结构呈现出杯状单体的典型二聚体,小角X射线散射实验证实了溶液中的二聚体。序列和结构比对显示,对活性重要的活性位点残基在Vs中是保守的尽管DapL的活性比其他DapL同源物的活性低。尽管二聚体界面占据了总表面积的18%,但对界面和活性位点有贡献的多个环(尤其是L1,L2和L5环)具有很高的移动性,这也许可以解释不稳定的二聚体和较低的催化活性。我们的动力学,生物物理和结构表征可用于告知抗生素的开发。











































 京公网安备 11010802027423号
京公网安备 11010802027423号