当前位置:
X-MOL 学术
›
J. Chin. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A charge‐separation‐type ionic solid composed of hexanuclear complexes with a macrocyclic tetragold(I) metalloligand
Journal of the Chinese Chemical Society ( IF 1.6 ) Pub Date : 2020-06-01 , DOI: 10.1002/jccs.202000163 Rycce S. Pratikha 1 , Takahiro Inoue 1 , Yuka Arai 1 , Tatsuhiro Kojima 1 , Nobuto Yoshinari 1 , Takumi Konno 1
Journal of the Chinese Chemical Society ( IF 1.6 ) Pub Date : 2020-06-01 , DOI: 10.1002/jccs.202000163 Rycce S. Pratikha 1 , Takahiro Inoue 1 , Yuka Arai 1 , Tatsuhiro Kojima 1 , Nobuto Yoshinari 1 , Takumi Konno 1
Affiliation
|
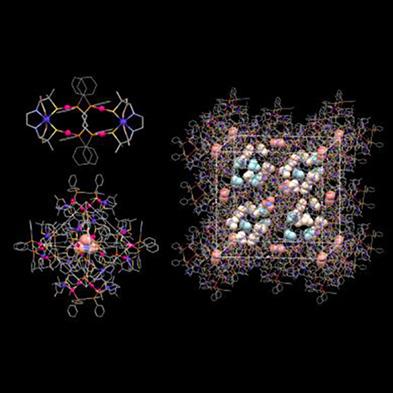
The reaction of Na[CoIII(d‐ebp)] (d‐H4ebp = N,N′‐ethylenebis[d‐penicillamine]) with [(AuICl)2(dppe)] (dppe = 1,2‐bis[diphenylphosphino]ethane) gave a cationic AuI4CoIII2 hexanuclear complex, [CoIII2(LAu4)]2+ ([1]2+), where [LAu4]4− is a cyclic tetragold(I) metalloligand with a 32‐membered ring, [AuI4(dppe)2(d‐ebp)2]4−. Complex [1]2+ crystallized with NO3− to produce a charge‐separation (CS)‐type ionic solid of [1](NO3)2. In [1](NO3)2, the complex cations are assembled to form cationic supramolecular hexamers of {[1]2+}6, which are closely packed in a face‐centered cubic (fcc) lattice structure. The nitrate anions of [1](NO3)2 were accommodated in hydrophilic and hydrophobic tetrahedral interstices of the fcc structure to form tetrameric and hexameric nitrate clusters of {NO3−}4 and {NO3−}6, respectively. An analogous CS‐type ionic solid formulated as [NiIICoIII(LAu4)](NO3) ([2](NO3)) was obtained when a 1:1 mixture of Na[CoIII(d‐ebp)] and [NiII(d‐H2ebp)] was reacted with [(AuICl)2(dppe)], accompanied by the conversion of the diamagnetic, square‐planar [NiII(d‐H2ebp)] to the paramagnetic, octahedral [NiII(d‐ebp)]2−. While the overall fcc structure in [2](NO3) was similar to that of [1](NO3)2, none of the nitrate anions were accommodated in any hydrophobic tetrahedral interstice, reflecting the difference in the complex charges between [1]2+ and [2]+.
中文翻译:

由六核配合物与大环四金(I)金属配体组成的电荷分离型离子固体
Na [Co III(d -ebp)](d -H 4 ebp = N,N'-亚乙基双[ d-青霉胺])与[(Au I Cl)2(dppe)](dppe = 1,2 -双[二苯基膦基乙烷])得到阳离子Au I 4 Co III 2六核络合物[Co III 2(L Au4)] 2 +([ 1 ] 2+),其中[L Au4 ] 4-是环状四金( I)具有32元环的金属配体,[Au I 4(dppe)2(d -ebp)2 ] 4−。配合物[ 1 ] 2+与NO结晶3 - ,以产生电荷分离(CS)型的[离子固体1 ](NO 3)2。在[ 1 ](NO 3)2中,复合阳离子组装形成{[ 1 ] 2+ } 6的阳离子超分子六聚体,它们紧密堆积在面心立方(fcc)晶格结构中。[ 1 ](NO 3)2的硝酸根阴离子被容纳在fcc结构的亲水性和疏水四面体空隙形成的{NO四聚体和六硝酸盐簇3 - } 4和{NO 3 - } 6,分别。当Na [Co III(d- ebp)1:1混合物时,获得了类似的CS型离子固体,其配制为[Ni II Co III(L Au4)](NO 3)([ 2 ](NO 3))。 ]和[Ni II(d -H 2 ebp)]与[(Au I Cl)2(dppe)],伴随着抗磁的方平面[Ni II(d -H 2 ebp)]转换为顺磁性的八面体[Ni II(d -ebp)] 2−。尽管[ 2 ](NO 3)的整体fcc结构与[ 1 ](NO 3)2的相似,但硝酸根阴离子均未容纳在任何疏水的四面体间隙中,反映了[ 1]之间的复合电荷的差异。] 2+和[ 2 ] +。
更新日期:2020-06-01
中文翻译:

由六核配合物与大环四金(I)金属配体组成的电荷分离型离子固体
Na [Co III(d -ebp)](d -H 4 ebp = N,N'-亚乙基双[ d-青霉胺])与[(Au I Cl)2(dppe)](dppe = 1,2 -双[二苯基膦基乙烷])得到阳离子Au I 4 Co III 2六核络合物[Co III 2(L Au4)] 2 +([ 1 ] 2+),其中[L Au4 ] 4-是环状四金( I)具有32元环的金属配体,[Au I 4(dppe)2(d -ebp)2 ] 4−。配合物[ 1 ] 2+与NO结晶3 - ,以产生电荷分离(CS)型的[离子固体1 ](NO 3)2。在[ 1 ](NO 3)2中,复合阳离子组装形成{[ 1 ] 2+ } 6的阳离子超分子六聚体,它们紧密堆积在面心立方(fcc)晶格结构中。[ 1 ](NO 3)2的硝酸根阴离子被容纳在fcc结构的亲水性和疏水四面体空隙形成的{NO四聚体和六硝酸盐簇3 - } 4和{NO 3 - } 6,分别。当Na [Co III(d- ebp)1:1混合物时,获得了类似的CS型离子固体,其配制为[Ni II Co III(L Au4)](NO 3)([ 2 ](NO 3))。 ]和[Ni II(d -H 2 ebp)]与[(Au I Cl)2(dppe)],伴随着抗磁的方平面[Ni II(d -H 2 ebp)]转换为顺磁性的八面体[Ni II(d -ebp)] 2−。尽管[ 2 ](NO 3)的整体fcc结构与[ 1 ](NO 3)2的相似,但硝酸根阴离子均未容纳在任何疏水的四面体间隙中,反映了[ 1]之间的复合电荷的差异。] 2+和[ 2 ] +。











































 京公网安备 11010802027423号
京公网安备 11010802027423号