当前位置:
X-MOL 学术
›
Chem. Biodivers.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A series of benzylidene linked to hydrazinecarbothioamide as tyrosinase inhibitorsSynthesis, biological evaluation and structure‐activity relationship
Chemistry & Biodiversity ( IF 2.3 ) Pub Date : 2020-08-01 , DOI: 10.1002/cbdv.202000285 Hona Hosseinpoor 1, 2 , Aida Iraji 1, 3 , Najmeh Edraki 1 , Somayeh Pirhadi 1 , Mahshid Attarroshan 1 , Mahsima Khoshneviszadeh 1 , Mehdi Khoshneviszadeh 1, 2
Chemistry & Biodiversity ( IF 2.3 ) Pub Date : 2020-08-01 , DOI: 10.1002/cbdv.202000285 Hona Hosseinpoor 1, 2 , Aida Iraji 1, 3 , Najmeh Edraki 1 , Somayeh Pirhadi 1 , Mahshid Attarroshan 1 , Mahsima Khoshneviszadeh 1 , Mehdi Khoshneviszadeh 1, 2
Affiliation
|
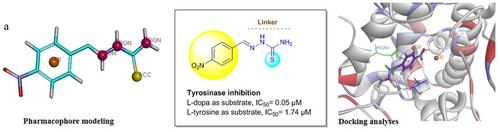
Tyrosinase is a type 3 copper enzyme responsible for skin pigmentation disorders, skin cancer, and enzymatic browning of vegetables and fruits. In the present article, 12 small molecules of benzylidene-hydrazinecarbothioamide were designed, synthesized and evaluated for their anti-tyrosinase activities followed by molecular docking and pharmacophore-based screening. Among synthesized thiosemicarbazone derivatives, 3d is the strongest inhibitor of mushroom tyrosinase with IC 50 of 0.05 µM which demonstrated a 128 fold increase in potency compared to the positive control. Kinetic studies also revealed mix type inhibition by 3d . Docking studies confirmed the complete fitting of the synthesized compounds into the tyrosinase active site. The results underline the potential of benzylidene hydrazinecarbothioamides as potent pharmacophore to extend the tyrosinase inhibition in drug discovery.
中文翻译:

一系列与肼碳硫酰胺相连的亚苄基作为酪氨酸酶抑制剂的合成、生物学评价和构效关系
酪氨酸酶是一种 3 型铜酶,可导致皮肤色素沉着障碍、皮肤癌以及蔬菜和水果的酶促褐变。在本文中,设计、合成了 12 个小分子亚苄基肼碳硫酰胺并评估了它们的抗酪氨酸酶活性,然后进行了分子对接和基于药效团的筛选。在合成的氨基硫脲衍生物中,3d 是最强的蘑菇酪氨酸酶抑制剂,IC 50 为 0.05 µM,与阳性对照相比,其效力提高了 128 倍。动力学研究还揭示了 3d 的混合类型抑制。对接研究证实合成的化合物完全适合酪氨酸酶活性位点。
更新日期:2020-08-01
中文翻译:

一系列与肼碳硫酰胺相连的亚苄基作为酪氨酸酶抑制剂的合成、生物学评价和构效关系
酪氨酸酶是一种 3 型铜酶,可导致皮肤色素沉着障碍、皮肤癌以及蔬菜和水果的酶促褐变。在本文中,设计、合成了 12 个小分子亚苄基肼碳硫酰胺并评估了它们的抗酪氨酸酶活性,然后进行了分子对接和基于药效团的筛选。在合成的氨基硫脲衍生物中,3d 是最强的蘑菇酪氨酸酶抑制剂,IC 50 为 0.05 µM,与阳性对照相比,其效力提高了 128 倍。动力学研究还揭示了 3d 的混合类型抑制。对接研究证实合成的化合物完全适合酪氨酸酶活性位点。











































 京公网安备 11010802027423号
京公网安备 11010802027423号