Waste Management ( IF 7.1 ) Pub Date : 2020-05-31 , DOI: 10.1016/j.wasman.2020.05.042 Shichao He 1 , Zhihong Liu 1
|
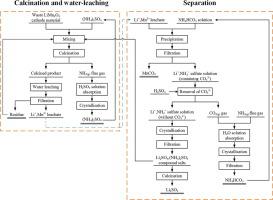
An efficient process is proposed for recovery of waste LiMn2O4 cathode material, which is one of the most commonly used cathode materials in LIBs. This report constitutes the precipitation thermodynamic analysis and separation experiments based on the water-leaching solutions during the processes of low-temperature calcination with (NH4)2SO4 and water-leaching. Precipitation thermodynamic analysis is undertaken to investigate the effects of initial concentration of the target solution, [N]T1, excess precipitant, and addition of (NH4)2SO4 on the manganese precipitation in the Mn2+–Li+–SO42−–NH3–NH4+–CO32−–H2O system. Moreover, the effects of initial concentration of the target solution, [N]T2, and excess precipitant on the lithium precipitation in the Li+–SO42−–NH3–NH4+–CO32−–H2O system are investigated. All these factors clearly influence the manganese and lithium precipitation, particularly the [N]T and the presence of excess precipitant in the system. The precipitation experimental results demonstrate that the optimal conditions are: a precipitation temperature of 35 °C; an excess coefficient of the precipitant of 2.4; the use of NHC-3 to precipitate the ML-3 solution; a maximum precipitation percentage of manganese of 99.96%; and an absence of Li2CO3 precipitation. The double-sulfate salts (Li(NH4)SO4 & (NH4)2SO4) evaporated and crystallised from the Li+/NH4+ solution are mixed with the waste LiMn2O4 cathode material for calcination and water leaching, for which the efficiencies of Li and Mn are 100% and 96.89%, respectively. The double-sulfate salts are calcined at 550 °C for 45 min to obtain the Li2SO4 product. Finally, the complete recovery and separation of Mn and Li in the waste LiMn2O4 cathode material are achieved.
中文翻译:

有效回收废旧正极材料LiMn2O4沉淀过程的热力学分析和分离实验。
提出了一种有效的方法来回收废LiMn 2 O 4阴极材料,该材料是LIB中最常用的阴极材料之一。该报告构成了在(NH 4)2 SO 4低温煅烧和水浸过程中基于水浸溶液的沉淀热力学分析和分离实验。进行了沉淀热力学分析,以研究目标溶液的初始浓度,[N] T1,过量沉淀剂和添加(NH 4)2 SO 4对Mn 2+中锰沉淀的影响。–Li + –SO 4 2− –NH 3 –NH 4 + –CO 3 2− –H 2 O系统。此外,目标溶液的初始浓度,[N] T2和过量的沉淀剂对Li + -SO 4 2-- NH 3 -NH 4 + -CO 3 2-- H 2 O系统中锂沉淀的影响被调查。所有这些因素显然都会影响锰和锂的沉淀,特别是[N] T以及系统中是否存在过多的沉淀剂。沉淀实验结果表明,最佳条件为:沉淀温度为35°C;温度为35°C。沉淀剂的过量系数为2.4;使用NHC-3沉淀ML-3溶液; 锰的最大沉淀百分比为99.96%;并且没有Li 2 CO 3沉淀。从Li + / NH 4 +溶液蒸发并结晶的双硫酸盐(Li(NH 4)SO 4和(NH 4)2 SO 4)与废LiMn 2 O 4混合用于煅烧和水浸的正极材料,其Li和Mn的效率分别为100%和96.89%。将双硫酸盐在550℃下煅烧45分钟以获得Li 2 SO 4产物。最后,完全回收和分离了废LiMn 2 O 4正极材料中的Mn和Li 。











































 京公网安备 11010802027423号
京公网安备 11010802027423号