当前位置:
X-MOL 学术
›
Hydrometallurgy
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Kinetics of two-step bioleaching of Ni and Co from iron rich-laterite using supernatant metabolites produced by Salinivibrio kushneri as halophilic bacterium
Hydrometallurgy ( IF 4.8 ) Pub Date : 2020-08-01 , DOI: 10.1016/j.hydromet.2020.105387 Marzieh Hosseini Nasab , Mohammad Noaparast , Hadi Abdollahi , Mohammad Ali Amoozegar
Hydrometallurgy ( IF 4.8 ) Pub Date : 2020-08-01 , DOI: 10.1016/j.hydromet.2020.105387 Marzieh Hosseini Nasab , Mohammad Noaparast , Hadi Abdollahi , Mohammad Ali Amoozegar
|
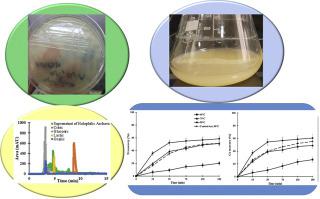
Abstract In the laterite leaching process using sulfuric acid, the addition of NaCl, for example at amount of 10% of solid weight, at 70 °C resulted the increase of nickel and cobalt recoveries up to 18.13 and 25.07%, respectively, and significant decrease in iron dissolution to 39.94% in leaching solution. Due to the role of salts in increasing the dissolutions of nickel and cobalt and decreasing iron dissolution, in this study, the possibility of using salt-friendly bacteria to extract nickel and cobalt from iron-rich laterite was investigated. The halophilic bacterium was isolated from Shorgaz Hamoon soil and grown in the medium with 10% NaCl. Results of HPLC analysis showed that gluconic acid (14.84 g/l), lactic acid (4.58 g/l) and citric acid (1.56 g/l) were the main organic acids in the supernatant metabolite of Salinivibrio kushneri as halophilic bacterium. Leaching experiments were performed at temperatures of 45 °C, 75 °C and 90 °C at pH of 0.5, stirring speed of 370 rpm, solid percentage of 6.67% w/v for 3 h. The results showed that recoveries of nickel and cobalt could reach to 58.40% and 60.6%, respectively after 3 h at 90 °C using supernatant metabolites of halophilic bacterium. Activation energies (Ea) for the chemical control model were 41.32 kJ/mol for nickel, and 40.24 kJ/mol for cobalt that these values indicated chemical control had more effect on the iron-rich laterite dissolution rate using supernatant metabolites produced by Salinivibrio kushneri than diffusion control.
中文翻译:

利用库什纳盐生菌产生的上清代谢物作为嗜盐菌从富铁红土中两步生物浸出镍和钴的动力学
摘要 在红土硫酸浸出过程中,70 ℃加入NaCl,例如固重的10%,镍和钴的回收率分别提高了18.13%和25.07%,显着下降。在浸出液中铁溶解到39.94%。由于盐类在增加镍和钴的溶解和减少铁的溶解方面的作用,本研究研究了使用盐友好细菌从富铁红土中提取镍和钴的可能性。从 Shorgaz Hamoon 土壤中分离出嗜盐细菌并在含有 10% NaCl 的培养基中生长。HPLC 分析结果表明,葡萄糖酸 (14.84 g/l)、乳酸 (4.58 g/l) 和柠檬酸 (1. 56 g/l) 是作为嗜盐细菌的库什纳盐菌上清液代谢物中的主要有机酸。浸出实验在 45°C、75°C 和 90°C 的温度下进行,pH 值为 0.5,搅拌速度为 370 rpm,固体百分比为 6.67% w/v,持续 3 小时。结果表明,使用嗜盐菌的上清代谢物,在 90 °C 下 3 h 后镍和钴的回收率分别达到 58.40% 和 60.6%。化学控制模型的活化能 (Ea) 镍为 41.32 kJ/mol,钴为 40.24 kJ/mol,这些值表明化学控制对使用由 Salinivibrio kushneri 产生的上清代谢物的富铁红土溶解速率的影响大于扩散控制。75°C 和 90°C,pH 值为 0.5,搅拌速度为 370 rpm,固体百分比为 6.67% w/v,持续 3 小时。结果表明,使用嗜盐菌的上清代谢物,在 90 °C 下 3 h 后镍和钴的回收率分别达到 58.40% 和 60.6%。化学控制模型的活化能 (Ea) 镍为 41.32 kJ/mol,钴为 40.24 kJ/mol,这些值表明化学控制对使用由 Salinivibrio kushneri 产生的上清代谢物的富铁红土溶解速率的影响大于扩散控制。75°C 和 90°C,pH 值为 0.5,搅拌速度为 370 rpm,固体百分比为 6.67% w/v,持续 3 小时。结果表明,使用嗜盐菌的上清代谢物,在 90 °C 下 3 h 后镍和钴的回收率分别达到 58.40% 和 60.6%。化学控制模型的活化能 (Ea) 镍为 41.32 kJ/mol,钴为 40.24 kJ/mol,这些值表明化学控制对使用由 Salinivibrio kushneri 产生的上清代谢物的富铁红土溶解速率的影响大于扩散控制。
更新日期:2020-08-01
中文翻译:

利用库什纳盐生菌产生的上清代谢物作为嗜盐菌从富铁红土中两步生物浸出镍和钴的动力学
摘要 在红土硫酸浸出过程中,70 ℃加入NaCl,例如固重的10%,镍和钴的回收率分别提高了18.13%和25.07%,显着下降。在浸出液中铁溶解到39.94%。由于盐类在增加镍和钴的溶解和减少铁的溶解方面的作用,本研究研究了使用盐友好细菌从富铁红土中提取镍和钴的可能性。从 Shorgaz Hamoon 土壤中分离出嗜盐细菌并在含有 10% NaCl 的培养基中生长。HPLC 分析结果表明,葡萄糖酸 (14.84 g/l)、乳酸 (4.58 g/l) 和柠檬酸 (1. 56 g/l) 是作为嗜盐细菌的库什纳盐菌上清液代谢物中的主要有机酸。浸出实验在 45°C、75°C 和 90°C 的温度下进行,pH 值为 0.5,搅拌速度为 370 rpm,固体百分比为 6.67% w/v,持续 3 小时。结果表明,使用嗜盐菌的上清代谢物,在 90 °C 下 3 h 后镍和钴的回收率分别达到 58.40% 和 60.6%。化学控制模型的活化能 (Ea) 镍为 41.32 kJ/mol,钴为 40.24 kJ/mol,这些值表明化学控制对使用由 Salinivibrio kushneri 产生的上清代谢物的富铁红土溶解速率的影响大于扩散控制。75°C 和 90°C,pH 值为 0.5,搅拌速度为 370 rpm,固体百分比为 6.67% w/v,持续 3 小时。结果表明,使用嗜盐菌的上清代谢物,在 90 °C 下 3 h 后镍和钴的回收率分别达到 58.40% 和 60.6%。化学控制模型的活化能 (Ea) 镍为 41.32 kJ/mol,钴为 40.24 kJ/mol,这些值表明化学控制对使用由 Salinivibrio kushneri 产生的上清代谢物的富铁红土溶解速率的影响大于扩散控制。75°C 和 90°C,pH 值为 0.5,搅拌速度为 370 rpm,固体百分比为 6.67% w/v,持续 3 小时。结果表明,使用嗜盐菌的上清代谢物,在 90 °C 下 3 h 后镍和钴的回收率分别达到 58.40% 和 60.6%。化学控制模型的活化能 (Ea) 镍为 41.32 kJ/mol,钴为 40.24 kJ/mol,这些值表明化学控制对使用由 Salinivibrio kushneri 产生的上清代谢物的富铁红土溶解速率的影响大于扩散控制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号