当前位置:
X-MOL 学术
›
Hydrometallurgy
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Behavior of rare earth, iron, and phosphorus during purification of rare earth sulfate leach solution using magnesium oxide
Hydrometallurgy ( IF 4.8 ) Pub Date : 2020-09-01 , DOI: 10.1016/j.hydromet.2020.105377 Shiliang Chen , Xiaowei Huang , Zongyu Feng , Yang Xu , Meng Wang , Chao Xia , Longsheng Zhao
Hydrometallurgy ( IF 4.8 ) Pub Date : 2020-09-01 , DOI: 10.1016/j.hydromet.2020.105377 Shiliang Chen , Xiaowei Huang , Zongyu Feng , Yang Xu , Meng Wang , Chao Xia , Longsheng Zhao
|
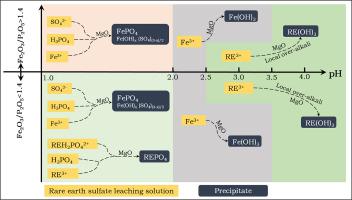
Abstract The largest rare earth (RE) reserves minerals of China is the bastnaesite (RE[CO3]F) and monazite ((RE, Th)[PO4]) mixed type RE ore in Bayan Obo, and 90% of this concentrate is processed by the sulfuric acid roasting method. However, during purification of RE sulfate leach solution with solid MgO, the RE loss is noticeable and accompanied by a mass of acid and alkali consumption and discharge of radioactive solid waste, which also causes environmental pollution. In this paper, the behavior of RE, Fe, and P during purification were investigated focusing on the effect of pH, concentration ratio of iron and phosphorus, RE concentration, and step-wise purification (based on different pH). The characterization of precipitated impurities by BET specific surface area, XRD, SEM and FTIR reveal the reaction sequence of RE loss and the formation of the precipitate. Two new factors, neglected previously, were found to be the main contributors to the production of large solid-waste:(1) sulfate which exists in the form of Fe(OH)x(SO4)(3-x)/2 accounts for 9 to 12% of the total weight of the precipitate; (2) higher solid content in the purification slurry at a relatively high pH range (pH = 2.5–4.5) significantly promotes the production of precipitate and higher loss of RE during purification. This paper proposes a two-step purification method (based on different middle pH point), step (i): a low pH purification stage in which most P, Fe(III) and H+ were removed with minimum loss of RE followed by filtration; step (ii), high pH purification stage in which the rest of Fe(III) was completely removed with less than 2% RE lost caused by low solid-to-liquid ratio consuming less MgO. Through two-step purification under the most suitable conditions, the RE recovery increased by 6% while MgO consumption and solid residue production decreased by 12% and 16%, respectively. It can greatly alleviate the environmental problems with just a minimal modification of the operation conditions and equipment in the proposed process.
更新日期:2020-09-01











































 京公网安备 11010802027423号
京公网安备 11010802027423号