当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Metal‐Free Synthesis of N‐Aryl Oxazolidin‐2‐Ones by the One‐Pot Reaction of Carbon Dioxide with N‐Aryl Aziridines
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-05-29 , DOI: 10.1002/adsc.202000175 Paolo Sonzini 1 , Caterina Damiano 1 , Daniela Intrieri 1 , Gabriele Manca 2 , Emma Gallo 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-05-29 , DOI: 10.1002/adsc.202000175 Paolo Sonzini 1 , Caterina Damiano 1 , Daniela Intrieri 1 , Gabriele Manca 2 , Emma Gallo 1
Affiliation
|
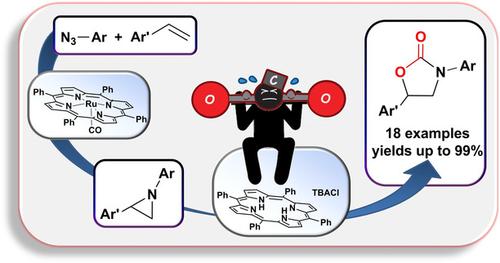
The cost‐effective TPPH2/TBACl‐catalyzed (TPPH2=dianion of tetraphenyl porphyrin; TBACl=tetrabutyl ammonium chloride) carbon dioxide cycloaddition to N‐aryl aziridines was successful in synthesizing N‐aryl oxazolidin‐2‐ones. A catalytic tandem reaction was also developed, in which N‐aryl aziridines were initially synthesized and then reacted with carbon dioxide without being purified. The procedure occurred with a very high atom economy, molecular nitrogen being the only by‐product of the entire tandem process. In addition, the mechanism of catalytic cycle was investigated by DFT calculations.
中文翻译:

二氧化碳与N-芳基氮丙啶的一锅法反应,无金属合成N-芳基恶唑烷-2-
具有成本效益的TPPH 2 / TBACl催化(TPPH 2 =四苯基卟啉的阴离子; TBACl =四丁基氯化铵)二氧化碳环化成N-芳基氮丙啶成功地合成了N-芳基恶唑烷二-2-酮。还开发了催化串联反应,其中最初合成了N-芳基氮丙啶,然后与二氧化碳反应而未纯化。该过程是在非常高的原子经济性下发生的,分子氮是整个串联过程的唯一副产物。另外,通过DFT计算研究了催化循环的机理。
更新日期:2020-07-29
中文翻译:

二氧化碳与N-芳基氮丙啶的一锅法反应,无金属合成N-芳基恶唑烷-2-
具有成本效益的TPPH 2 / TBACl催化(TPPH 2 =四苯基卟啉的阴离子; TBACl =四丁基氯化铵)二氧化碳环化成N-芳基氮丙啶成功地合成了N-芳基恶唑烷二-2-酮。还开发了催化串联反应,其中最初合成了N-芳基氮丙啶,然后与二氧化碳反应而未纯化。该过程是在非常高的原子经济性下发生的,分子氮是整个串联过程的唯一副产物。另外,通过DFT计算研究了催化循环的机理。











































 京公网安备 11010802027423号
京公网安备 11010802027423号