Journal of Proteomics ( IF 2.8 ) Pub Date : 2020-05-30 , DOI: 10.1016/j.jprot.2020.103848 Guibin Wang 1 , Yuqian Wang 2 , Lishan Zhang 2 , Qilan Cai 2 , Yuexu Lin 2 , Ling Lin 2 , Xiangmin Lin 3
|
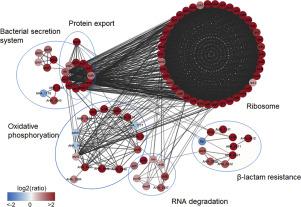
Our previous studies have profiled lysine acetylation and succinylation modifications in Aeromonas hydrophila protein and have found that CobB may be involved in lysine deacylation; however, its effects on bacterial biological function are still unknown. In this study, a data-independent acquisition (DIA)-based proteomics method was used to compare the protein abundance between cob-deleted mutants and wild-type strains. Of the total 2385 identified proteins, 385 were found to have increased abundance, while only 46 showed decreased abundance. Data analysis revealed that many proteins in six metabolic pathways, ribosome, the bacterial secretion system, protein export, RNA degradation, beta-Lactam resistance and oxidative phosphorylation, were affected by the deletion of cobB. Some proteins, such as outer membrane proteins, the two-component regulatory systems and transcriptional factor, were also regulated by cobB. The following phenotype assays confirmed that the ΔcobB mutant produced more biofilm, migrated farther in soft agar, and was more sensitive to oxidative stress than its WT parent. Taken together, the results presented herein provide insights into the behaviors of sirtuin protein CobB in bacteria and demonstrate its important biological functions in A. hydrophila.
Biological significance
The sirtuin protein CobB play crucial roles on lysine deacylation, such as desuccinylation and deacetylation in many bacterial species, while the intrinsic behavior of CobB on bacteria remains elusive. The current DIA-based quantitative proteomics analysis showed that the deletion of A. hydrophila cobB significantly affect the intracellular biological processes. Further phenotype assays validated proteomics results. Overall, our data further confirmed the important roles of CobB on the complex protein-protein interaction network regulation in A. hydrophila.
中文翻译:

蛋白质组学分析揭示了嗜水气单胞菌Sirtuin CobB对生物学功能的影响。
我们以前的研究已经描述了嗜水气单胞菌蛋白中赖氨酸的乙酰化和琥珀酰化修饰,并发现CobB可能参与了赖氨酸的脱酰作用。然而,其对细菌生物学功能的影响仍然未知。在这项研究中,使用了一个与数据无关的采集(DIA)基蛋白质组学方法之间进行比较的蛋白质丰度穗轴-deleted突变体和野生型菌株。在鉴定出的2385种蛋白质中,有385种的丰度增加,而只有46种的丰度降低。数据分析表明,六个代谢途径中的许多蛋白质,如核糖体,细菌分泌系统,蛋白质输出,RNA降解,β-内酰胺抗性和氧化磷酸化,都受到缺失的影响。科布。一些蛋白质,例如外膜蛋白质,两组分调节系统和转录因子,也受到cobB的调节。以下表型分析证实了ΔcobB突变体比野生WT亲本产生更多的生物膜,在软琼脂中迁移更远,并且对氧化应激更敏感。两者合计,结果在此给出见解沉默调节蛋白科布在细菌的行为,并证明其重要的生物学功能嗜水气单。
生物学意义
瑟土因蛋白CobB在赖氨酸脱酰作用中起着关键作用,例如许多细菌物种中的脱琥珀酰化和脱乙酰化作用,而CobB在细菌上的固有行为仍然难以捉摸。当前基于DIA的定量蛋白质组学分析表明,嗜水链球菌cobB的缺失显着影响细胞内的生物学过程。进一步的表型分析验证了蛋白质组学结果。总的来说,我们的数据进一步证实的重要作用科布在复杂的蛋白质-蛋白质相互作用网络监管嗜水气单。











































 京公网安备 11010802027423号
京公网安备 11010802027423号