Journal of Fluorine Chemistry ( IF 1.7 ) Pub Date : 2020-05-30 , DOI: 10.1016/j.jfluchem.2020.109577 Tamara A. Vaganova , Yurij V. Gatilov , Denis P. Pishchur , Evgenij V. Malykhin
|
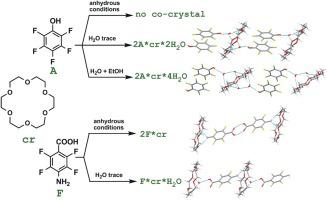
Potential of polyfluoroaromatic compounds containing OH, COOH, and mixed H-donor functions as co-formers in co-crystallization with 18-crown-6 ether was explored. Pentafluorophenol, 1,3- and 1,4-dihydroxytetrafluorobenzenes and pentafluorobenzoic acid form with 18-crown-6 hydrated co-crystals containing one water molecule per H-donor group upon contact of the solutions with atmospheric moisture; pentafluorophenol gives a co-crystal with double the amount of water in aqueous EtOH. Aminotetrafluorobenzyl alcohol and aminotetrafluorobenzoic acid are capable of forming anhydrous 1:1 and 2:1 co-crystals with 18-crown-6. In the crystalline hydrates, co-formers molecules are connected via water-mediated synthon [C(O)]OH⋯O(H)
H⋯Ocr. According to quantum chemical calculations, the participation of each water linker molecule in H-bonding increases the stability of the supramolecular structure by about 100 kJ mol−1. Associates of bifunctional arenes exhibit H-bonded 1D assembly as an only structure-determining motif; molecules of monofunctional arenes in the co-crystals are bound via πF⋯πF stacking to give the H-bonded/π-stacked 1D assembly. The calculated π-stacking energy depends on the nature of the H-donor function (NH2, OH, COOH), decreasing with a decrease in the molecule dipole moment.
中文翻译:

多氟羟基苯酚和羧基苯作为与18冠6醚自组装的新型H受体:合成,超分子结构和共晶体的稳定性
探索了含有OH,COOH和混合的H-供体的多氟芳族化合物在与18-冠-6醚共结晶中作为共形成剂的潜力。当溶液与大气中的水分接触时,五氟苯酚,1,3-和1,4-二羟基四氟苯与五氟苯甲酸与18个皇冠6个水合共晶体形成一个水分子,每个H供体基团含有一个水分子。五氟苯酚产生的共晶体与在EtOH水溶液中的水量加倍。氨基四氟苄醇和氨基四氟苯甲酸能够与18-crown-6形成无水1:1和2:1共结晶。在结晶水合物中,共形成物分子通过水介导的合成子[C(O)] O H⋯O(H)
H⋯O cr连接。根据量子化学计算,每个水连接分子参与H键合将超分子结构的稳定性提高约100kJ mol -1。双官能团的缔合体表现出H键合的1D组装作为唯一的结构决定基元。在共晶的单官能芳烃的分子通过结合π ˚F ⋯π ˚F堆叠,得到H-粘结/π堆叠1D组件。计算出的π-堆积能取决于H-给体功能(NH 2,OH,COOH)的性质,随着分子偶极矩的减小而减小。











































 京公网安备 11010802027423号
京公网安备 11010802027423号