International Journal of Hydrogen Energy ( IF 8.1 ) Pub Date : 2020-05-29 , DOI: 10.1016/j.ijhydene.2020.04.257 Yufa Feng , Huize Wang , Xiaodong Chen , Fei Lv , Yuanzhong Li , Yongmei Zhu , Chujia Xu , Xibin Zhang , Hui-Ru Liu , Hao Li
|
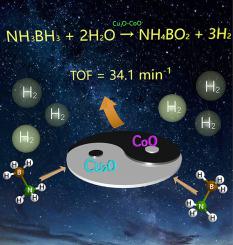
The development of inexpensive and high performing catalysts for ammonia borane (NH3BH3) hydrolysis is crucial for hydrogen production. In our research, a high-performance plate-like Cu2O–CoO nanocomposite catalyst for NH3BH3 hydrolysis has been developed for the first time. In the hydrolytic reaction, both Cu2O and CoO are separately inactive, while Cu2O–CoO nanoplates show a high turnover frequency of 34.1 molhydrogen min−1 molcat−1, which is attributed to the synergistic effect between Cu2O and CoO. It is interesting to discover that the induction time for the hydrolytic reaction is reduced to null when a small amount of Cu2O is introduced into CoO. The reaction kinetics of NH3BH3 hydrolysis catalyzed by Cu2O–CoO is also investigated. This work may provide other researchers some valuable insights into designing inexpensive and synergistic catalysts with enhanced catalytic activity for NH3BH3 hydrolysis for hydrogen production.
中文翻译:

简单合成具有增强催化活性的氨硼烷水解制氢的Cu 2 O–CoO纳米板
廉价,高性能的氨硼烷(NH 3 BH 3)水解催化剂的开发对于制氢至关重要。在我们的研究中,首次开发了用于NH 3 BH 3水解的高性能板状Cu 2 O–CoO纳米复合催化剂。在水解反应中,Cu 2 O和CoO分别处于非活性状态,而Cu 2 O–CoO纳米板的氢转换频率很高,为34.1 mol氢 min -1 mol cat -1,这归因于Cu 2之间的协同作用O和CoO。有趣的是,当将少量Cu 2 O引入CoO时,水解反应的诱导时间减少为零。还研究了Cu 2 O–CoO催化的NH 3 BH 3水解反应动力学。这项工作可能会为其他研究人员提供一些有价值的见识,以帮助他们设计出廉价且具有协同作用的催化剂,以增强对生成氨的NH 3 BH 3水解的催化活性。










































 京公网安备 11010802027423号
京公网安备 11010802027423号