当前位置:
X-MOL 学术
›
Chin. J. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanochemical redox-based synthesis of highly porous Co Mn1-O catalysts for total oxidation
Chinese Journal of Catalysis ( IF 15.7 ) Pub Date : 2020-12-01 , DOI: 10.1016/s1872-2067(20)63635-x Jiafeng Bao , Hao Chen , Shize Yang , Pengfei Zhang
Chinese Journal of Catalysis ( IF 15.7 ) Pub Date : 2020-12-01 , DOI: 10.1016/s1872-2067(20)63635-x Jiafeng Bao , Hao Chen , Shize Yang , Pengfei Zhang
|
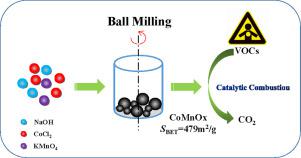
Abstract A mechanochemical redox reaction between KMnO4 and CoCl2 was developed to obtain a CoxMn1–xOy catalyst with a specific surface area of 479 m2 g−1, which was higher than that obtained using a co-precipitation (CP) method (34 m2 g−1), sol-gel (SG) method (72 m2 g−1), or solution redox process (131 m2 g−1). During catalytic combustion, this CoxMn1–xOy catalyst exhibited better activity (T100 for propylene = ~200 °C) than the control catalysts obtained using the SG (325 °C) or CP (450 °C) methods. The mechanical action, mainly in the form of kinetic energy and frictional heating, may generate a high degree of interstitial porosity, while the redox reaction could contribute to good dispersion of cobalt and manganese species. Moreover, the as-prepared CoxMn1–xOy catalyst worked well in the presence of water vapor (H2O 4.2%, >60 h) or SO2 (100 ppm) and at high temperature (400 °C, > 60 h). The structure MnO2·(CoOOH)2.93 was suggested for the current CoxMn1–xOy catalyst. This catalyst could be extended to the total oxidation of other typical hydrocarbons (T90 = 150 °C for ethanol, T90 = 225 °C for acetone, T90 = 250 °C for toluene, T90 = 120 °C for CO, and T90 = 540 °C for CH4). Scale-up of the synthesis of CoxMn1–xOy catalyst (1 kg) can be achieved via ball milling, which may provide a potential strategy for real world catalysis.
中文翻译:

基于机械化学氧化还原的高多孔 Co Mn1-O 催化剂合成用于全氧化
摘要 通过 KMnO4 和 CoCl2 之间的机械化学氧化还原反应,获得了比表面积为 479 m2 g-1 的 CoxMn1-xOy 催化剂,比使用共沉淀 (CP) 方法获得的比表面积 (34 m2 g- 1)、溶胶-凝胶 (SG) 方法(72 m2 g-1)或溶液氧化还原工艺(131 m2 g-1)。在催化燃烧过程中,这种 CoxMn1-xOy 催化剂表现出比使用 SG (325 °C) 或 CP (450 °C) 方法获得的对照催化剂更好的活性(丙烯的 T100 = ~200 °C)。主要以动能和摩擦加热形式的机械作用可能会产生高度的间隙孔隙,而氧化还原反应可能有助于钴和锰物种的良好分散。此外,所制备的 CoxMn1-xOy 催化剂在水蒸气(H2O 4.2%,> 60 小时)或 SO2 (100 ppm) 和高温(400 °C,> 60 小时)。目前的 CoxMn1-xOy 催化剂结构为 MnO2·(CoOOH)2.93。这种催化剂可以扩展到其他典型碳氢化合物的总氧化(乙醇的 T90 = 150 °C,丙酮的 T90 = 225 °C,甲苯的 T90 = 250 °C,CO 的 T90 = 120 °C,T90 = 540 ℃,CH4)。CoxMn1-xOy 催化剂(1 kg)合成的放大可以通过球磨实现,这可能为现实世界的催化提供一种潜在的策略。
更新日期:2020-12-01
中文翻译:

基于机械化学氧化还原的高多孔 Co Mn1-O 催化剂合成用于全氧化
摘要 通过 KMnO4 和 CoCl2 之间的机械化学氧化还原反应,获得了比表面积为 479 m2 g-1 的 CoxMn1-xOy 催化剂,比使用共沉淀 (CP) 方法获得的比表面积 (34 m2 g- 1)、溶胶-凝胶 (SG) 方法(72 m2 g-1)或溶液氧化还原工艺(131 m2 g-1)。在催化燃烧过程中,这种 CoxMn1-xOy 催化剂表现出比使用 SG (325 °C) 或 CP (450 °C) 方法获得的对照催化剂更好的活性(丙烯的 T100 = ~200 °C)。主要以动能和摩擦加热形式的机械作用可能会产生高度的间隙孔隙,而氧化还原反应可能有助于钴和锰物种的良好分散。此外,所制备的 CoxMn1-xOy 催化剂在水蒸气(H2O 4.2%,> 60 小时)或 SO2 (100 ppm) 和高温(400 °C,> 60 小时)。目前的 CoxMn1-xOy 催化剂结构为 MnO2·(CoOOH)2.93。这种催化剂可以扩展到其他典型碳氢化合物的总氧化(乙醇的 T90 = 150 °C,丙酮的 T90 = 225 °C,甲苯的 T90 = 250 °C,CO 的 T90 = 120 °C,T90 = 540 ℃,CH4)。CoxMn1-xOy 催化剂(1 kg)合成的放大可以通过球磨实现,这可能为现实世界的催化提供一种潜在的策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号