当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Organocatalytic asymmetric Friedel–Crafts alkylation/hemiketalization/lactonization cascade reactions: highly enantioselective synthesis of furo[2,3-b]benzofuranones
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2020-05-29 , DOI: 10.1039/d0qo00475h Kunhua Xu 1, 2, 3, 4, 5 , Wenming Chen 5, 6, 7, 8 , Xu Chen 1, 2, 3, 4, 5 , Biao Wang 1, 2, 3, 4, 5 , Jun Huang 3, 4, 9, 10, 11 , Xu Tian 1, 2, 3, 4, 5
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2020-05-29 , DOI: 10.1039/d0qo00475h Kunhua Xu 1, 2, 3, 4, 5 , Wenming Chen 5, 6, 7, 8 , Xu Chen 1, 2, 3, 4, 5 , Biao Wang 1, 2, 3, 4, 5 , Jun Huang 3, 4, 9, 10, 11 , Xu Tian 1, 2, 3, 4, 5
Affiliation
|
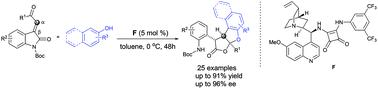
An efficient asymmetric organocatalytic Friedel–Crafts alkylation/hemiketalization/lactonization cascade reaction of 3-ylidene oxindoles with 2-naphthols and activated phenols has been developed. In the presence of 5 mol% of a bifunctional squaramide catalyst, this scalable cascade reaction affords a series of novel furo[2,3-b]benzofuranone derivatives with three adjacent stereogenic centers in good to excellent yields (up to 91%) and with high stereoselectivities (up to 96% ee, >20 : 1 d.r.). Moreover, this cascade reaction provides a distinct lactonization process of amide functional groups under mild reaction conditions to access the diversely decorated furo[2,3-b]benzofuranones of considerable importance to natural product synthesis and medicinal chemistry.
中文翻译:

有机催化不对称Friedel-Crafts烷基化/半缩酮化/内酯化级联反应:呋喃并[2,3-b]苯并呋喃酮的高度对映选择性合成
已经开发出了一种有效的不对称有机催化的3-亚甲基羟吲哚与2-萘酚和活化酚的不对称Friedel-Crafts烷基化/半缩酮化/内酯化级联反应。在5摩尔%的双功能方酰胺催化剂的存在下,这种可扩展的级联反应可提供一系列新颖的呋喃[2,3- b ]苯并呋喃酮衍生物,其具有三个相邻的立体异构中心,产率高至优异(高达91%),并且具有高立体选择性(高达96%ee,> 20:1 dr)。此外,该级联反应在温和的反应条件下提供了酰胺官能团的独特内酯化过程,从而可以访问对天然产物合成和药物化学相当重要的各种装饰的呋喃[2,3- b ]苯并呋喃酮。
更新日期:2020-06-30
中文翻译:

有机催化不对称Friedel-Crafts烷基化/半缩酮化/内酯化级联反应:呋喃并[2,3-b]苯并呋喃酮的高度对映选择性合成
已经开发出了一种有效的不对称有机催化的3-亚甲基羟吲哚与2-萘酚和活化酚的不对称Friedel-Crafts烷基化/半缩酮化/内酯化级联反应。在5摩尔%的双功能方酰胺催化剂的存在下,这种可扩展的级联反应可提供一系列新颖的呋喃[2,3- b ]苯并呋喃酮衍生物,其具有三个相邻的立体异构中心,产率高至优异(高达91%),并且具有高立体选择性(高达96%ee,> 20:1 dr)。此外,该级联反应在温和的反应条件下提供了酰胺官能团的独特内酯化过程,从而可以访问对天然产物合成和药物化学相当重要的各种装饰的呋喃[2,3- b ]苯并呋喃酮。











































 京公网安备 11010802027423号
京公网安备 11010802027423号