当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Anaerobic Dissolution Rates of U(IV)-Oxide by Abiotic and Nitrate-Dependent Bacterial Pathways.
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2020-05-29 , DOI: 10.1021/acs.est.0c01019 Maria P Asta 1 , Harry R Beller 2, 3 , Peggy A O'Day 1, 4
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2020-05-29 , DOI: 10.1021/acs.est.0c01019 Maria P Asta 1 , Harry R Beller 2, 3 , Peggy A O'Day 1, 4
Affiliation
|
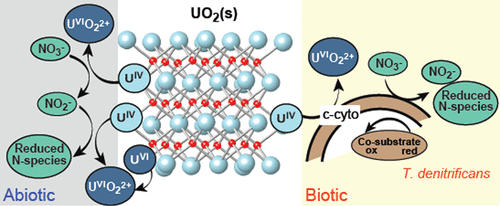
The long-term stability of U(IV) solid phases in anaerobic aquifers depends upon their reactivity in the presence of oxidizing chemical species and microbial catalysts. We performed flow-through column experiments under anaerobic conditions to investigate the mechanisms and dissolution rates of biogenic, noncrystalline UO2(s) by chemical oxidants (nitrate and/or nitrite) or by Thiobacillus denitrificans, a widespread, denitrifying, chemolithoautotrophic model bacterium. Dissolution rates of UO2(s) with dissolved nitrite were approximately 5 to 10 times greater than with nitrate alone. In the presence of wild-type T. denitrificans and nitrate, UO2(s) dissolution rates were similar to those of abiotic experiments with nitrite (from 1.15 × 10–14 to 4.94 × 10–13 mol m–2 s–1). Experiments with a T. dentrificans mutant strain defective in U(IV) oxidation supported microbially mediated U(IV) oxidation. X-ray absorption spectroscopy (XAS) analysis of post-reaction solids showed the presence of mononuclear U(VI) species rather than a solid U(VI) phase. At steady-state U release, kinetic and spectroscopic results suggest detachment of oxidized U(VI) from the UO2(s) surface as the rate-determining step rather than electron transfer or ion diffusion. Under anaerobic conditions, production of nitrite by nitrate-reducing microorganisms and enzymatically catalyzed, nitrate-dependent U(IV) oxidation are likely dual processes by which reduced U solids may be oxidized and mobilized in the aqueous phase.
中文翻译:

非生物和硝酸盐依赖性细菌途径对U(IV)-氧化物的厌氧溶解速率
U(IV)固相在厌氧含水层中的长期稳定性取决于它们在氧化性化学物质和微生物催化剂存在下的反应性。我们在厌氧条件下进行了流式色谱柱实验,以研究化学氧化剂(硝酸盐和/或亚硝酸盐)或反硝化硫杆菌(一种广泛的,反硝化的化学自养型细菌)引起的生物,非晶态UO 2(s)的机理和溶解速率。UO 2(s)在溶解的亚硝酸盐中的溶解速率大约是单独硝酸盐的5至10倍。在野生型反硝化锥虫和硝酸盐存在下,UO 2(s)的溶出速率与使用亚硝酸盐的非生物实验相似(从1.15×10 –14到4.94×10 –13 mol m –2 s –1)。具有U(IV)氧化缺陷的T. dentrificans突变菌株的实验支持微生物介导的U(IV)氧化。反应后固体的X射线吸收光谱(XAS)分析表明存在单核U(VI)物种而不是固态U(VI)相。在稳态的U释放下,动力学和光谱结果表明氧化的U(VI)从UO 2脱离(s)表面作为速率确定步骤,而不是电子转移或离子扩散。在厌氧条件下,由还原硝酸盐的微生物生产亚硝酸盐和酶催化的依赖硝酸盐的U(IV)氧化很可能是双重过程,通过还原过程,还原的U固体可以被氧化并在水相中动员。
更新日期:2020-07-07
中文翻译:

非生物和硝酸盐依赖性细菌途径对U(IV)-氧化物的厌氧溶解速率
U(IV)固相在厌氧含水层中的长期稳定性取决于它们在氧化性化学物质和微生物催化剂存在下的反应性。我们在厌氧条件下进行了流式色谱柱实验,以研究化学氧化剂(硝酸盐和/或亚硝酸盐)或反硝化硫杆菌(一种广泛的,反硝化的化学自养型细菌)引起的生物,非晶态UO 2(s)的机理和溶解速率。UO 2(s)在溶解的亚硝酸盐中的溶解速率大约是单独硝酸盐的5至10倍。在野生型反硝化锥虫和硝酸盐存在下,UO 2(s)的溶出速率与使用亚硝酸盐的非生物实验相似(从1.15×10 –14到4.94×10 –13 mol m –2 s –1)。具有U(IV)氧化缺陷的T. dentrificans突变菌株的实验支持微生物介导的U(IV)氧化。反应后固体的X射线吸收光谱(XAS)分析表明存在单核U(VI)物种而不是固态U(VI)相。在稳态的U释放下,动力学和光谱结果表明氧化的U(VI)从UO 2脱离(s)表面作为速率确定步骤,而不是电子转移或离子扩散。在厌氧条件下,由还原硝酸盐的微生物生产亚硝酸盐和酶催化的依赖硝酸盐的U(IV)氧化很可能是双重过程,通过还原过程,还原的U固体可以被氧化并在水相中动员。











































 京公网安备 11010802027423号
京公网安备 11010802027423号