当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Influence of Surface Compositions on the Reactivity of Pyrite toward Aqueous U(VI).
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2020-05-29 , DOI: 10.1021/acs.est.0c01854 Bin Ma 1 , Alejandro Fernandez-Martinez 1 , Mingliang Kang 2 , Kaifeng Wang 1, 3 , Aled R Lewis 4 , Thierry G G Maffeis 4 , Nathaniel Findling 1 , Eduardo Salas-Colera 5, 6 , Delphine Tisserand 1 , Sarah Bureau 1 , Laurent Charlet 1
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2020-05-29 , DOI: 10.1021/acs.est.0c01854 Bin Ma 1 , Alejandro Fernandez-Martinez 1 , Mingliang Kang 2 , Kaifeng Wang 1, 3 , Aled R Lewis 4 , Thierry G G Maffeis 4 , Nathaniel Findling 1 , Eduardo Salas-Colera 5, 6 , Delphine Tisserand 1 , Sarah Bureau 1 , Laurent Charlet 1
Affiliation
|
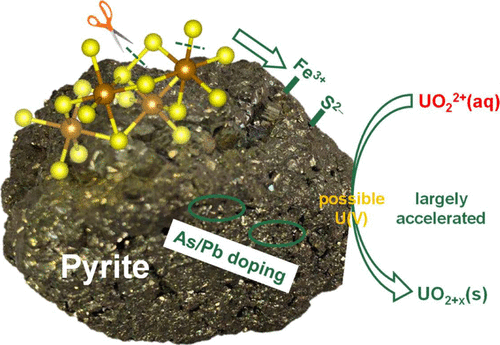
Pyrite plays a significant role in governing the mobility of toxic uranium in an anaerobic environment via an oxidation–reduction process occurring at the mineral–water interface, but the factors influencing the reaction kinetics remain poorly understood. In this study, natural pyrites with different impurities (Pb, As, and Si) and different surface pretreatments were used to react with aqueous U(VI) from pH ∼3.0 to ∼9.5. Both aqueous and solid results indicated that freshly crushed pyrites, which do have more surface Fe2+/Fe3+ and S2– sites that were generated from breakage of Fe(S)–S bonds during ball milling, exhibited a much stronger reactivity than those treated with acid washing. Besides, U(VI) reduction which involves the possible intermediate U(V) and the formation of hyperstoichiometric UO2+x(s) was found to preferentially occur at Pb- and As-rich spots on the pyrite surface, suggesting that the incorporated impurities could act as reactive sites because of the generation of lattice defects and galena- and arsenopyrite-like local configurations. These reactive surface sites can be removed by acid washing, leaving a pyrite surface nearly inert toward aqueous U(VI). Thus, reactivity of pyrite toward U(VI) is largely governed by its surface compositions, which provides an insight into the chemical behavior of both pyrite and uranium in various environments.
中文翻译:

表面成分对黄铁矿对U(VI)水溶液反应性的影响。
黄铁矿在矿物质-水界面发生的氧化还原过程,在控制有氧铀在厌氧环境中的迁移中起着重要作用,但是影响反应动力学的因素仍然知之甚少。在这项研究中,使用具有不同杂质(Pb,As和Si)和不同表面预处理的天然黄铁矿与U(VI)水溶液在pH约3.0至9.5之间进行反应。水性和固态结果均表明,刚粉碎的黄铁矿的确具有更多的表面Fe 2+ / Fe 3+和S 2–球磨过程中由Fe(S)-S键断裂产生的位点比经酸洗处理的位点具有更强的反应性。此外,U(VI)还原涉及可能的中间U(V)和超化学计量UO 2+ x的形成发现(一种或多种)杂质优先出现在黄铁矿表面富Pb和As的点上,这表明掺入的杂质可能由于晶格缺陷和方铅矿和毒砂状局部结构的生成而充当反应位点。这些反应性表面部位可通过酸洗除去,而使黄铁矿表面几乎对U(VI)水溶液呈惰性。因此,黄铁矿对U(VI)的反应性主要受其表面组成的控制,这可洞察黄铁矿和铀在各种环境中的化学行为。
更新日期:2020-07-07
中文翻译:

表面成分对黄铁矿对U(VI)水溶液反应性的影响。
黄铁矿在矿物质-水界面发生的氧化还原过程,在控制有氧铀在厌氧环境中的迁移中起着重要作用,但是影响反应动力学的因素仍然知之甚少。在这项研究中,使用具有不同杂质(Pb,As和Si)和不同表面预处理的天然黄铁矿与U(VI)水溶液在pH约3.0至9.5之间进行反应。水性和固态结果均表明,刚粉碎的黄铁矿的确具有更多的表面Fe 2+ / Fe 3+和S 2–球磨过程中由Fe(S)-S键断裂产生的位点比经酸洗处理的位点具有更强的反应性。此外,U(VI)还原涉及可能的中间U(V)和超化学计量UO 2+ x的形成发现(一种或多种)杂质优先出现在黄铁矿表面富Pb和As的点上,这表明掺入的杂质可能由于晶格缺陷和方铅矿和毒砂状局部结构的生成而充当反应位点。这些反应性表面部位可通过酸洗除去,而使黄铁矿表面几乎对U(VI)水溶液呈惰性。因此,黄铁矿对U(VI)的反应性主要受其表面组成的控制,这可洞察黄铁矿和铀在各种环境中的化学行为。











































 京公网安备 11010802027423号
京公网安备 11010802027423号