当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Emerging Potassium Metal Anodes: Perspectives on Control of the Electrochemical Interfaces.
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2020-05-28 , DOI: 10.1021/acs.accounts.0c00099 Pengcheng Liu 1 , David Mitlin 1
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2020-05-28 , DOI: 10.1021/acs.accounts.0c00099 Pengcheng Liu 1 , David Mitlin 1
Affiliation
|
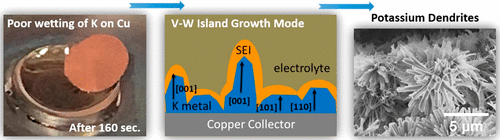
Potassium metal serves as the anode in emerging potassium metal batteries (KMBs). It also serves as the counter electrode for potassium ion battery (KIB) half-cells, with its reliable performance being critical for assessing the working electrode material. This first-of-its-kind critical Account focuses on the dual challenge of controlling the potassium metal-substrate and the potassium metal–electrolyte interface so as to prevent dendrites. The discussion begins with a comparison of the physical and chemical properties of K metal anodes versus the much oft studied Li and Na metal anodes. Based on established descriptions for root causes of dendrites, the problem should be less severe for K than for Li or Na, while in fact the opposite is observed. The key reason that the K metal surface rapidly becomes dendritic in common electrolytes is its unstable solid electrolyte interphase (SEI). An unstable SEI layer is defined as being non-self-passivating. No SEI is perfectly stable during cycling, and all SEI structures are heterogeneous both vertically and horizontally relative to the electrolyte interface. The difference between a “stable” and an “unstable” SEI may be viewed as the relative degree to which during cycling it thickens and becomes further heterogeneous. The unstable SEI on K metal leads to a number of interrelated problems, such as low cycling Coulombic efficiency (CE), a severe impedance rise, large overpotentials, and possibly electrical shorting, all of which have been reported to occur as early as in the first 10 plating/stripping cycles. Many of the traditional “interface fixes” employed for Li and Na metal anodes, such as various artificial SEIs, surface membranes, barrier layers, secondary separators, etc., have not been attempted or optimized for the case of K. This is an important area for further exploration, with an understanding that success may come harder with K than with Li due to K-based SEI reactivity with both ether and ester solvents.
中文翻译:

新兴的钾金属阳极:电化学界面控制的观点。
金属钾是新兴的钾金属电池(KMB)的阳极。它也用作钾离子电池(KIB)半电池的对电极,其可靠的性能对于评估工作电极材料至关重要。这是第一个关键帐户,着重于控制钾金属底物和钾金属-电解质界面以防止枝晶的双重挑战。讨论首先将K金属阳极的物理和化学性质与经过大量研究的Li和Na金属阳极进行比较。根据树突的根本原因的既定描述,K的问题应不如Li或Na严重,而实际上却相反。普通电解质中K金属表面迅速变为树枝状的关键原因是其不稳定的固体电解质中间相(SEI)。不稳定的SEI层定义为非自钝性。没有SEI在循环过程中是完全稳定的,并且所有SEI结构相对于电解质界面在垂直和水平方向上都是异质的。“稳定”和“不稳定”的SEI之间的差异可以看作是循环过程中SEI变厚并变得更加不均匀的相对程度。钾金属上不稳定的SEI会导致许多相互关联的问题,例如循环库仑效率(CE)低,严重的阻抗升高,过大的电势以及可能的电气短路,所有这些据报道都发生在早期。前10个电镀/剥离周期。
更新日期:2020-05-28
中文翻译:

新兴的钾金属阳极:电化学界面控制的观点。
金属钾是新兴的钾金属电池(KMB)的阳极。它也用作钾离子电池(KIB)半电池的对电极,其可靠的性能对于评估工作电极材料至关重要。这是第一个关键帐户,着重于控制钾金属底物和钾金属-电解质界面以防止枝晶的双重挑战。讨论首先将K金属阳极的物理和化学性质与经过大量研究的Li和Na金属阳极进行比较。根据树突的根本原因的既定描述,K的问题应不如Li或Na严重,而实际上却相反。普通电解质中K金属表面迅速变为树枝状的关键原因是其不稳定的固体电解质中间相(SEI)。不稳定的SEI层定义为非自钝性。没有SEI在循环过程中是完全稳定的,并且所有SEI结构相对于电解质界面在垂直和水平方向上都是异质的。“稳定”和“不稳定”的SEI之间的差异可以看作是循环过程中SEI变厚并变得更加不均匀的相对程度。钾金属上不稳定的SEI会导致许多相互关联的问题,例如循环库仑效率(CE)低,严重的阻抗升高,过大的电势以及可能的电气短路,所有这些据报道都发生在早期。前10个电镀/剥离周期。











































 京公网安备 11010802027423号
京公网安备 11010802027423号