当前位置:
X-MOL 学术
›
ChemPhysChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Molecular Motions and Interactions in Aqueous Solutions of Thymosin-β4 , Stabilin CTD and Their 1 : 1 Complex, Studied by 1 H-NMR Spectroscopy.
ChemPhysChem ( IF 2.3 ) Pub Date : 2020-06-08 , DOI: 10.1002/cphc.202000264 M Bokor 1 , Á Tantos 2 , A Mészáros 2 , B Jenei 2 , R Haminda 3 , P Tompa 2, 4 , K Tompa 1
ChemPhysChem ( IF 2.3 ) Pub Date : 2020-06-08 , DOI: 10.1002/cphc.202000264 M Bokor 1 , Á Tantos 2 , A Mészáros 2 , B Jenei 2 , R Haminda 3 , P Tompa 2, 4 , K Tompa 1
Affiliation
|
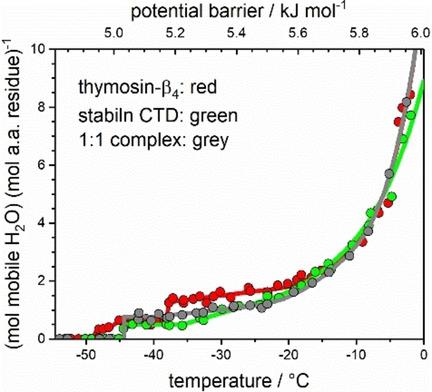
Wide‐line 1H NMR measurements were extended and all results were interpreted in a thermodynamics‐based new approach on aqueous solutions of thymosin‐β4 (Tβ4), stabilin cytoplasmic domain (CTD), and their 1 : 1 complex. Energy distributions of potential barriers controlling the motion of protein‐bound water molecules were determined. Heterogeneous and homogeneous regions were found in the protein‐water interface. The measure of heterogeneity of this interface gives quantitative value for the portion of disordered parts in the protein. Ordered structural elements were found extending up to ∼20 % of the individual whole proteins. About 40 % of the binding sites of free Tβ4 get involved in bonds holding the complex together. The complex has the most heterogeneous solvent accessible surface (SAS) in terms of protein‐water interactions. The complex is more disordered than Tβ4 or stabilin CTD. The greater SAS area of the complex is interpreted as a clear sign of its open structure.
中文翻译:

胸腺素-β4,稳定素CTD及其1:1配合物在水溶液中的分子运动和相互作用,通过1 H-NMR光谱研究。
宽行1次1 H NMR测定是扩展和所有结果被解释在胸腺素β的水溶液基于热力学-新方法4(Tβ 4),stabilin胞质结构域(CTD),和它们的1:1的复合物。确定了控制蛋白质结合水分子运动的势垒的能量分布。在蛋白质-水界面中发现了异质和均质区域。该界面异质性的度量给出了蛋白质中无序部分的定量值。发现有序的结构元件延伸至单个完整蛋白质的约20%。关于免费Tβ的结合位点的40%4参与将复合体结合在一起的债券。就蛋白质与水的相互作用而言,该复合物具有最不均匀的溶剂可及表面(SAS)。复杂的是比Tβ更加无序4或stabilin CTD。该综合体的较大SAS面积被解释为其开放结构的明显标志。
更新日期:2020-06-08
中文翻译:

胸腺素-β4,稳定素CTD及其1:1配合物在水溶液中的分子运动和相互作用,通过1 H-NMR光谱研究。
宽行1次1 H NMR测定是扩展和所有结果被解释在胸腺素β的水溶液基于热力学-新方法4(Tβ 4),stabilin胞质结构域(CTD),和它们的1:1的复合物。确定了控制蛋白质结合水分子运动的势垒的能量分布。在蛋白质-水界面中发现了异质和均质区域。该界面异质性的度量给出了蛋白质中无序部分的定量值。发现有序的结构元件延伸至单个完整蛋白质的约20%。关于免费Tβ的结合位点的40%4参与将复合体结合在一起的债券。就蛋白质与水的相互作用而言,该复合物具有最不均匀的溶剂可及表面(SAS)。复杂的是比Tβ更加无序4或stabilin CTD。该综合体的较大SAS面积被解释为其开放结构的明显标志。











































 京公网安备 11010802027423号
京公网安备 11010802027423号