Tetrahedron ( IF 2.1 ) Pub Date : 2020-05-29 , DOI: 10.1016/j.tet.2020.131315 Zhen He , Tony Biremond , Gregory J.P. Perry , David J. Procter
|
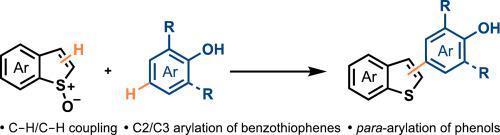
C2 and C3 substituted benzothiophenes are common structures in medicinal and materials chemistry. The cross-coupling of phenols with benzothiophenes is a useful route towards these important molecules. In this report we reveal an efficient C–H/C–H-type cross-coupling of benzothiophenes, activated as their S-oxides, with phenols to give C2/C3 arylated benzothiophenes. Whereas previous reports describe cross-coupling at the ortho-position between phenols and sulfoxides, this procedure allows para-functionalization of phenols that typically have their ortho positions blocked.
中文翻译:

对位-耦合与C2 /酚的C3-取代苯并噻吩小号-oxides
C2和C3取代的苯并噻吩是药物和材料化学中的常见结构。苯酚与苯并噻吩的交叉偶联是通往这些重要分子的有用途径。在本报告中,我们揭示了苯并噻吩类,活化作为其的有效C-H / C-H型交叉耦合小号-oxides,与酚,得到C2 / C3芳基化的苯并噻吩。尽管先前的报道描述了在酚和亚砜之间在邻位处的交叉偶联,但是该方法允许通常被邻位封端的酚的对官能化。







































 京公网安备 11010802027423号
京公网安备 11010802027423号