Materials Today Energy ( IF 9.0 ) Pub Date : 2020-05-29 , DOI: 10.1016/j.mtener.2020.100436 J. Wang , X. Ge , L. Shao , J. Zhang , D. Peng , G. Zou , H. Hou , W. Deng , S. Xu , X. Ji , W. Zhang
|
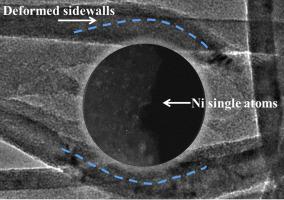
N-doped carbon nanotube-supported Ni/NiO hybrid structures with Ni/NiO crystalline interfaces (NiO/Ni@NCNTs) were investigated as electrocatalysts for the hydrogen evolution reaction (HER) in alkaline solution, featuring an overpotential of 87.5 mV at a current density of 10 mA cm−2. During catalyzation, reaction-driven transformation of Ni/NiO hybrid structures into Ni single atoms and clusters was observed on deformed NCNTs. These single atoms (clusters) remained anchored even after harsh washing in acid, manifesting that NiO/Ni@NCNTs may be used as a platform for stabilised site-isolated catalysis in practical electrolytic processes. Surface characterization shows that metallic Ni was shown to mainly originate from Ni atoms bonded to pyridinic N atoms of the carbon skeleton. As shown by theoretical calculations, the Gibbs free energy ΔG (H∗) of the H adsorption was minimised when a Ni atom was adjacent to an O atom on a Ni (111) surface, which indicated that metallic Ni partially covered with NiO was energetically favourable for the HER.
中文翻译:

Ni / NiO杂化结构的反应驱动转变为Ni单原子
研究了N掺杂的碳纳米管支撑的具有Ni / NiO晶体界面(NiO / Ni @ NCNTs)的Ni / NiO杂化结构作为碱性溶液中氢分解反应(HER)的电催化剂,在当前电流下的过电势为87.5 mV。密度为10 mA cm -2。在催化过程中,在变形的NCNTs上观察到反应驱动的Ni / NiO杂化结构转变为Ni单原子和簇。这些单原子(簇)即使在酸中剧烈洗涤后仍保持锚定状态,表明NiO / Ni @ NCNT可以用作实际电解过程中稳定的位点隔离催化的平台。表面表征表明金属镍主要来自与碳骨架的吡啶N原子键合的Ni原子。如理论计算所示,当Ni原子与Ni(111)表面上的O原子相邻时,H吸附的吉布斯自由能ΔG(H *)最小,这表明部分被NiO覆盖的金属Ni充满能量对她有利。











































 京公网安备 11010802027423号
京公网安备 11010802027423号