当前位置:
X-MOL 学术
›
Mater. Today Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Defect-induced anisotropic surface reactivity and ion transfer processes of anatase nanoparticles
Materials Today Chemistry ( IF 6.7 ) Pub Date : 2020-09-01 , DOI: 10.1016/j.mtchem.2020.100290 M.-S. Lee , K.S. Han , J. Lee , Y. Shin , T.C. Kaspar , Y. Chen , M.H. Engelhard , K.T. Mueller , V. Murugesan
Materials Today Chemistry ( IF 6.7 ) Pub Date : 2020-09-01 , DOI: 10.1016/j.mtchem.2020.100290 M.-S. Lee , K.S. Han , J. Lee , Y. Shin , T.C. Kaspar , Y. Chen , M.H. Engelhard , K.T. Mueller , V. Murugesan
|
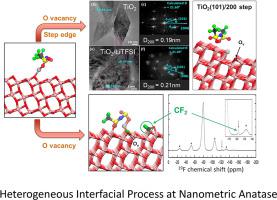
Abstract Surface reactivity and ion transfer processes of anatase TiO2 nanocrystals were studied using lithium bis(trifluoromethylsulfone)imide (LiTFSI) as a probing molecule. Analysis of synthesized anatase TiO2 by electron microscopy reveals aggregated nanoparticles (average size ~8 nm) with significant defects (holes and cracks). With the introduction of LiTFSI salt, the Li+-adsorption propensity towards the surface along the anatase (100) step edge plane is evident in both x-ray diffraction (XRD) and high-resolution transmission electron microscopy (HRTEM) analysis. Ab initio molecular dynamics (AIMD) analysis corroborates the site-preferential interaction of Li+ cations with oxygen vacancies and the thermodynamically favorable transport through the (100) step edge plane. Using 7Li nuclear magnetic resonance (NMR) chemical shift and relaxometry measurements, the presence of Li+ cations near the interface between TiO2 and the bulk LiTFSI phase was identified, and subsequent diffusion properties were analyzed. The lower activation energy derived from NMR analysis reveals enhanced mobility of Li+ cations along the surface, in good agreement with AIMD calculations. On the other hand, the TFSI– anion interaction with defect sites leads to CF3 bond dissociation and subsequent generation of carbonyl fluoride-type species. The multimodal spectroscopic analysis including NMR, electron paramagnetic resonance (EPR), and x-ray photoelectron spectroscopy (XPS) confirms the decomposition of TFSI– anions near the anatase surface. The reaction mechanism and electronic structure of interfacial constituents were simulated using AIMD calculations. Overall, this work demonstrates the role of defects at the anatase nanoparticle surface on charge transfer and interfacial reaction processes.
中文翻译:

缺陷诱导的锐钛矿纳米颗粒的各向异性表面反应性和离子转移过程
摘要 以双(三氟甲基砜)亚胺锂(LiTFSI)为探测分子,研究了锐钛矿型TiO2纳米晶的表面反应性和离子转移过程。通过电子显微镜分析合成的锐钛矿 TiO2 显示聚集的纳米颗粒(平均尺寸 ~8 nm)具有显着缺陷(孔洞和裂缝)。随着 LiTFSI 盐的引入,在 X 射线衍射 (XRD) 和高分辨率透射电子显微镜 (HRTEM) 分析中,沿着锐钛矿 (100) 台阶边缘平面向表面吸附 Li+ 的倾向是显而易见的。从头算分子动力学 (AIMD) 分析证实了 Li+ 阳离子与氧空位的位点优先相互作用以及通过 (100) 台阶边缘平面的热力学有利传输。使用 7Li 核磁共振 (NMR) 化学位移和弛豫测量,确定了在 TiO2 和本体 LiTFSI 相之间的界面附近存在 Li+ 阳离子,并分析了随后的扩散特性。来自 NMR 分析的较低活化能表明 Li+ 阳离子沿表面的迁移率增强,与 AIMD 计算结果一致。另一方面,TFSI-阴离子与缺陷位点的相互作用导致 CF3 键解离和随后生成碳酰氟类物质。包括 NMR、电子顺磁共振 (EPR) 和 X 射线光电子能谱 (XPS) 在内的多峰光谱分析证实了锐钛矿表面附近 TFSI-阴离子的分解。使用AIMD计算模拟了界面成分的反应机理和电子结构。总的来说,这项工作证明了锐钛矿纳米颗粒表面的缺陷对电荷转移和界面反应过程的作用。
更新日期:2020-09-01
中文翻译:

缺陷诱导的锐钛矿纳米颗粒的各向异性表面反应性和离子转移过程
摘要 以双(三氟甲基砜)亚胺锂(LiTFSI)为探测分子,研究了锐钛矿型TiO2纳米晶的表面反应性和离子转移过程。通过电子显微镜分析合成的锐钛矿 TiO2 显示聚集的纳米颗粒(平均尺寸 ~8 nm)具有显着缺陷(孔洞和裂缝)。随着 LiTFSI 盐的引入,在 X 射线衍射 (XRD) 和高分辨率透射电子显微镜 (HRTEM) 分析中,沿着锐钛矿 (100) 台阶边缘平面向表面吸附 Li+ 的倾向是显而易见的。从头算分子动力学 (AIMD) 分析证实了 Li+ 阳离子与氧空位的位点优先相互作用以及通过 (100) 台阶边缘平面的热力学有利传输。使用 7Li 核磁共振 (NMR) 化学位移和弛豫测量,确定了在 TiO2 和本体 LiTFSI 相之间的界面附近存在 Li+ 阳离子,并分析了随后的扩散特性。来自 NMR 分析的较低活化能表明 Li+ 阳离子沿表面的迁移率增强,与 AIMD 计算结果一致。另一方面,TFSI-阴离子与缺陷位点的相互作用导致 CF3 键解离和随后生成碳酰氟类物质。包括 NMR、电子顺磁共振 (EPR) 和 X 射线光电子能谱 (XPS) 在内的多峰光谱分析证实了锐钛矿表面附近 TFSI-阴离子的分解。使用AIMD计算模拟了界面成分的反应机理和电子结构。总的来说,这项工作证明了锐钛矿纳米颗粒表面的缺陷对电荷转移和界面反应过程的作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号