Journal of Water Process Engineering ( IF 6.3 ) Pub Date : 2020-05-28 , DOI: 10.1016/j.jwpe.2020.101369 Duong Huu Huy , Emily Seelen , Van Liem-Nguyen
|
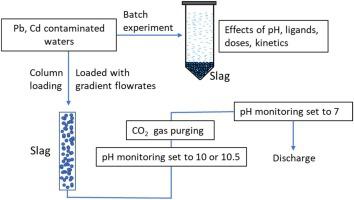
In this work, steel slag was shown to be a successful filter material for the removal of lead and cadmium from natural waters with a removal efficiency of >95 %, even under acidic conditions (pH 3.5). Overall, precipitation was found to be the main removal mechanism over adsorption with a tight relationship with the final matrix pH. Batch experiments showed fast removal kinetics with >98 % removal efficiency for lead and cadmium in 5 and 15 min, respectively. The removal kinetics followed a pseudo second-order reaction (R2 = 0.999), and the K2 was calculated to be 927 h−1 and 204 h−1 for lead and cadmium, respectively. A strong interference for the removal was observed with ethylenediaminetetraacetic acid (EDTA) but slightly with fulvic and humic acids, and non-significant with polymeric acid e.g. isosaccharinic acid. In addition, the column experiment showed spontaneous removal kinetics for lead and cadmium with a contact time of 3.6–36 seconds depending on loading flow-rates. Column experiments at similar liquid to solid ratios showed a high removal capacity with gradient over isocratic solution loading. The high removal efficiencies in combination with limited hazardous metals leaching led to the conclusion that slag is an appropriate filter material for treating contaminated waters. Moreover, the slag treated solutions with alkaline conditions showed reacted efficiently with CO2 in flue gas. Consequently, the pH of the treated solution is neutralized making it easier to waste and this reaction may contribute to reducing CO2 gas emissions.
中文翻译:

废旧金属回收产生的不锈钢渣对污水中镉和铅离子的去除机理。
在这项工作中,钢渣被证明是一种成功的过滤材料,即使在酸性条件下(pH 3.5),也能从天然水中去除铅和镉,去除效率> 95%。总体而言,发现沉淀是吸附的主要去除机理,与最终基质的pH密切相关。批处理实验显示,在5分钟和15分钟内,铅和镉的快速去除动力学具有> 98%的去除效率。去除动力学遵循假想的二级反应(R 2 = 0.999),计算得出的K 2为927 h -1和204 h -1分别用于铅和镉。用乙二胺四乙酸(EDTA)观察到对去除的强烈干扰,但对富里酸和腐殖酸有轻微的干扰,而对聚合酸(如异糖精酸)无明显影响。另外,柱实验显示了铅和镉的自发去除动力学,接触时间为3.6–36秒,具体取决于加载流速。在相似的液/固比下进行的柱实验显示出较高的去除能力,且梯度超过等度溶液负载。高去除效率与有限的有害金属浸出相结合得出的结论是,炉渣是处理污水的合适过滤材料。此外,经碱处理的炉渣溶液显示出与CO 2有效反应在烟气中。因此,处理溶液的pH值被中和,使其更容易浪费,并且该反应可有助于减少CO 2气体排放。











































 京公网安备 11010802027423号
京公网安备 11010802027423号