Chemical Physics ( IF 2.0 ) Pub Date : 2020-05-29 , DOI: 10.1016/j.chemphys.2020.110859 Yaogang Zhang , Xi Yu , Guangjun Tian , Yanying Zhu
|
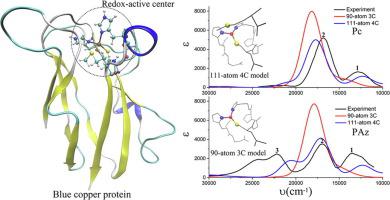
We present first-principles calculations on the geometrical structures and optical absorption properties of redox-active centers in two types of blue copper proteins, plastocyanin (Pc) and pseudoazurin (PAz). Two four-coordination (4C) cluster models consisting 111-atom were constructed based on the crystal structures of the two proteins. The optimized configurations of the proposed models took on highly identical geometry as the crystal structures and the calculated absorption spectra of both models acceptably reproduced the corresponding experimental spectra. Based on spectral simulations, we were able to confirm the indispensable role of axial ligand methionine (Met) in both proteins. The difference in the absorption spectroscopy of the two types of proteins was caused by the changes to the ground state wave functions which originate mainly from the different coordination structures of the redox-active center, especially the different Cu-SMet bond lengths. The effects of density functionals and the size of basis sets were also tested.
中文翻译:

蓝铜蛋白氧化还原活性中心的结构和光谱学的第一性原理研究
我们提出关于两种类型的蓝色铜蛋白,质体蓝蛋白(Pc)和假天青素(PAz)中的氧化还原活性中心的几何结构和光吸收特性的第一性原理计算。基于两种蛋白质的晶体结构,构建了由111个原子组成的两个四配位(4C)簇模型。所提出的模型的优化配置具有与晶体结构高度相同的几何形状,并且两个模型的计算吸收光谱可接受地再现了相应的实验光谱。基于光谱模拟,我们能够确认轴向配体蛋氨酸(Met)在这两种蛋白质中必不可少的作用。达到粘结长度。还测试了密度泛函和基集大小的影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号