当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Disulphide-mediated site-directed modification of proteins.
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2020-05-27 , DOI: 10.1039/d0ob00861c Thorbjørn Nielsen 1 , Anders Märcher 2 , Zuzana Drobňáková 3 , Michal Hučko 3 , Milan Štengl 3 , Vojtěch Balšánek 3 , Charlotte Wiberg 4 , Per F Nielsen 4 , Thomas E Nielsen 4 , Kurt V Gothelf 2 , Emiliano Cló 4
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2020-05-27 , DOI: 10.1039/d0ob00861c Thorbjørn Nielsen 1 , Anders Märcher 2 , Zuzana Drobňáková 3 , Michal Hučko 3 , Milan Štengl 3 , Vojtěch Balšánek 3 , Charlotte Wiberg 4 , Per F Nielsen 4 , Thomas E Nielsen 4 , Kurt V Gothelf 2 , Emiliano Cló 4
Affiliation
|
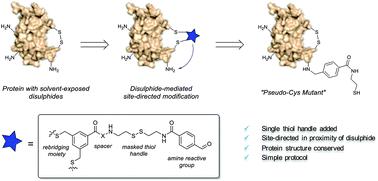
Methods for chemical modification of native proteins in a controlled fashion are in high demand. Here, a novel protocol that exploits bifunctional reagents for transient targeting of solvent exposed disulphides to direct the introduction of a single exogenous reactive thiol handle at a lysine side chain has been developed. The protocol has successfully been applied to functionalize six different Fabs and human growth hormone.
中文翻译:

二硫键介导的蛋白质的定点修饰。
迫切需要以受控方式对天然蛋白质进行化学修饰的方法。在这里,已经开发出一种新颖的方案,该方案利用双功能试剂来瞬时靶向暴露于溶剂的二硫化物,以指导在赖氨酸侧链上引入单个外源性反应性硫醇提手。该协议已成功应用于功能化六个不同的Fab和人类生长激素。
更新日期:2020-07-01
中文翻译:

二硫键介导的蛋白质的定点修饰。
迫切需要以受控方式对天然蛋白质进行化学修饰的方法。在这里,已经开发出一种新颖的方案,该方案利用双功能试剂来瞬时靶向暴露于溶剂的二硫化物,以指导在赖氨酸侧链上引入单个外源性反应性硫醇提手。该协议已成功应用于功能化六个不同的Fab和人类生长激素。











































 京公网安备 11010802027423号
京公网安备 11010802027423号