当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
In situ structural evolution of the multi-site alloy electrocatalyst to manipulate the intermediate for enhanced water oxidation reaction
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2020-05-28 , DOI: 10.1039/d0ee00755b Bingliang Wang 1, 2, 3, 4, 5 , Kangning Zhao 6, 7, 8, 9, 10 , Zhuo Yu 1, 2, 3, 4, 5 , Congli Sun 6, 7, 8, 9, 10 , Zhuo Wang 1, 2, 3, 4, 5 , Ningning Feng 1, 2, 3, 4, 5 , Liqiang Mai 6, 7, 8, 9, 10 , Yonggang Wang 1, 2, 3, 4, 5 , Yongyao Xia 1, 2, 3, 4, 5
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2020-05-28 , DOI: 10.1039/d0ee00755b Bingliang Wang 1, 2, 3, 4, 5 , Kangning Zhao 6, 7, 8, 9, 10 , Zhuo Yu 1, 2, 3, 4, 5 , Congli Sun 6, 7, 8, 9, 10 , Zhuo Wang 1, 2, 3, 4, 5 , Ningning Feng 1, 2, 3, 4, 5 , Liqiang Mai 6, 7, 8, 9, 10 , Yonggang Wang 1, 2, 3, 4, 5 , Yongyao Xia 1, 2, 3, 4, 5
Affiliation
|
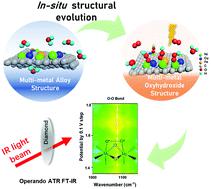
Investigating the reaction mechanism and the rational design of highly efficient electrocatalysts for the oxygen evolution reaction play a key role in renewable energy applications. Here, we report a homogeneous multi-metal-site oxyhydroxide electrocatalyst (consisting of Fe doped NiOOH and Cu doped NiOOH) obtained by in situ electrochemical dealloying of the multi-metal-site alloy (consisting of FeNi3 and NiCu alloys). The in situ structural evolution process manipulates the intermediate and enhances the water oxidation performance. After dealloying, the electrochemically dealloyed catalyst exhibits a small overpotential at large current density (250 mV at 100 mA cm−2), low Tafel slope (34 mV dec−1), remarkably increased ECSA (8-fold larger than before), and superior durability for 200 h at 100 mA cm−2. This electrocatalyst presents one of the best performances among all reported transition metal-based electrocatalysts, and is even superior to the benchmark RuO2. Operando ATR FT-IR reveals that the electrochemically dealloyed electrocatalyst could manipulate the reaction path based on direct O2 evolution mechanism (DOEM) and facilitate the formation of O–O bonds. This fundamental understanding will contribute to the identification and design of the active structure of oxygen evolution electrocatalysts.
中文翻译:

多部位合金电催化剂的原位结构演变,以控制中间体以增强水氧化反应
研究氧气释放反应的反应机理和高效电催化剂的合理设计在可再生能源应用中起着关键作用。在这里,我们报告了一种通过多金属部位合金(由FeNi 3和NiCu合金组成)的原位电化学脱合金获得的均质多金属部位的羟基氧化物电催化剂(由Fe掺杂的NiOOH和Cu掺杂的NiOOH组成)。该原位构造演化过程操纵中间并增强了水氧化性能。脱合金后,经电化学脱合金的催化剂在大电流密度下(100 mA cm -2时为250 mV,在Tafel斜率较低(34 mV dec -1)下表现出较小的过电位),ECSA显着增加(比以前大8倍),并在100 mA cm -2的条件下200小时具有出色的耐久性。该电催化剂是所有已报道的过渡金属基电催化剂中性能最好的一种,甚至优于基准RuO 2。Operando ATR FT-IR表明,电化学脱合金的电催化剂可以基于直接的O 2逸出机理(DOEM)操纵反应路径并促进O-O键的形成。这种基本的理解将有助于氧析出电催化剂活性结构的鉴定和设计。
更新日期:2020-07-15
中文翻译:

多部位合金电催化剂的原位结构演变,以控制中间体以增强水氧化反应
研究氧气释放反应的反应机理和高效电催化剂的合理设计在可再生能源应用中起着关键作用。在这里,我们报告了一种通过多金属部位合金(由FeNi 3和NiCu合金组成)的原位电化学脱合金获得的均质多金属部位的羟基氧化物电催化剂(由Fe掺杂的NiOOH和Cu掺杂的NiOOH组成)。该原位构造演化过程操纵中间并增强了水氧化性能。脱合金后,经电化学脱合金的催化剂在大电流密度下(100 mA cm -2时为250 mV,在Tafel斜率较低(34 mV dec -1)下表现出较小的过电位),ECSA显着增加(比以前大8倍),并在100 mA cm -2的条件下200小时具有出色的耐久性。该电催化剂是所有已报道的过渡金属基电催化剂中性能最好的一种,甚至优于基准RuO 2。Operando ATR FT-IR表明,电化学脱合金的电催化剂可以基于直接的O 2逸出机理(DOEM)操纵反应路径并促进O-O键的形成。这种基本的理解将有助于氧析出电催化剂活性结构的鉴定和设计。











































 京公网安备 11010802027423号
京公网安备 11010802027423号